Chloroform Formula: Preparation, Properties and Uses
Chloroform is a tri chloro derivative of methane. The structure of Chloroform is achieved by substituting three chlorine atoms in place of three hydrogen atoms in a methane molecule.
Chloroform Formula :- CHCl₃
Chloroform: Method of Preparation
Chloroform reaction: Chloroform is obtained by heating ethyl alcohol, aceteldihyde or any methyl kiton such as acetone and methyl ethyl kiton with an aqueous solution of chlorine and an alkali. This reaction is called chloroform reaction.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
Reaction of ethyl alcohol chlorine and NaOH solution
Chloroform Preparation :
Cl2 + H2O → 2HCl + O
C2H5OH + O → CH3CHO + H2O
CH3CHO + 3Cl2 → CCl3CHO + 3HCl
CCl3CHO + NaOH → CHCl3 + HCOONa
[HCl + NaOH → NaCl + H2O] x 5
Final Reaction :
C2H5OH + 4Cl2 + 6NaOH → CHCl3 + NCOONa + 5NaCl + 5H2O
Reaction of aceteldihyde chlorine and NaOH solution
Chloroform Preparation :
CH3CHO + 3Cl2 → CCl3CHO + 3HCl
CCl3CHO + NaOH → CHCl3 + HCOONa
[HCl + NaOH → NaCl + H2O] x 3
Final Reaction :
CH3CHO + 3Cl2 + 4NaOH → CHCl3 + HCOONa + 3NaCl
Reaction of acetone chlorine and NaOH solution
Chloroform
Preparation:
CH3COCH3
+ 3Cl2 → CCl3COCH3 + 3HCl
CCl3COCH3
+ NaOH → CHCl3 + CH3COONa
[HCl + NaOH →
NaCl + H2O] x 3
Final Reaction :
CH3COCH3
+ 3Cl2 + 4NaOH → CHCl3 + CH3COONa + 3NaCl + 3H3O
From ethyl alcohol or Acetone: In the laboratory, ethyl alcohol or acetone is heated with bleaching powder and water to form chloroform.
Bleaching powder of ethyl alcohol and reaction with water:
This action takes place in the following terms.
Firstly chlorine are obtained by the reaction of water with bleaching powder.
CaOCl2 + H2O → Ca(OH)2 + Cl2
The chlorine is then oxidized to ethyl alcohol to form acetaldehyde.

The chlorination of acetaldehyde made in the above reaction gets trichloro-aldehyde (chloral).
CH3CHO + 3Cl2 → CCl3CHO + 3HCl
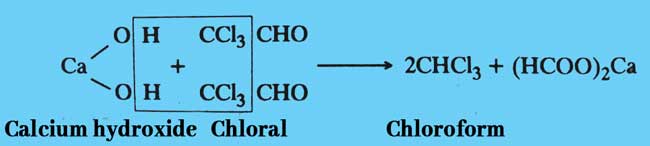
Finally, chloroform is obtained by reaction chloral with calcium hydroxide.
Take a mixture made of a mixture of about 100 grams of bleaching powder and 200 ml of water into a round-bottomed flask. Add 25 ml ethanol or acetone to it and attach it to the condenser.
The condenser is connected to the receiver by the edapter. Put the flask on the sand heat and heat it slowly. The chloroform is distilled and collected under water kept in the receptor.
Chloroform is isolated from this mixture by a separating funnel. Water and ethanol or acetone are present as impurities.
To purify it, first wash it with dilute caustic soda solution and then wash it with water, finally pure chroloform is achieved at around 61 C by the efficient distillation method.
- Electric Cell : E.M.F., Terminal Potential, Internal Resistance
- How Transistor Works : PNP and NPN Transistors
- How p-n Junction Diode works : Forward and Reverse Biasing
- Semiconductors : How Semiconductor works and Types
- X-Rays – Production, Properties, Wavelength and Uses
- Daily use Chemical Compounds and Their Properties
Industrial method: Chroloform can also be made in large quantities by heating a mixture of ethyl alcohol or acetone, water and bleaching powder, similar to the laboratory method of making Chroloform.
In another method, partial reduction of carbon tetra chlorine by iron powder and water vapor is done to make it more plentiful.
3Fe + 4H2O → Fe3O4 + 8H
CCl4 + 2H → CHCl3 + HCl
Chloral hydrate: Pure chloroform is obtained after distillation of a mixture of chloral hydrate and concentrated solution of sodium or potessium hydroxide.
NaOH + CCl3CH(OH)2 → CHCl3 + HCOONa + H2O
Physical Properties of Chloroform
Chloroform is a colorless liquid. Its boiling point is 61°C. Its smell is sweet and distinct. Breathing in its vapors leads to unconsciousness. It is almost immiscible in water but miscible in ether and alcohol. It is heavier than water, so when added to water, it forms a lower surface. It is a good solvent for oils, fats, waxes, rubber, etc.
Chemical Properties of Chloroform
Oxidation: In the presence of sunlight and air, chloroform is gradually oxidized to form carbonyl chloride.
2CHCl3 + O2 → 2COCl2 + 2HCl
Phosgene is a highly toxic gas. To prevent oxidation of chloroform
Read more about Phosgene Visit this page
1) It is kept in blue or brown bottles so that the sunlight is absorbed by the colored glass of the bottles.
2) Stoppers must be placed on the mouth of these bottles so that chloroform is not exposed to excess air. To protect chloroform, add 1% ethyl alcohol to it. It acts as a negative catalyst for the above reaction. Additionally, it reacts with phosgene phosgene to form harmless ethyl carbonate.
COCl2 + 2C2H5OH → (C2H5)2CO3 + 3HCl
Reduction: It gets methylene chloride, methyl chloride and methane by being oxidized by nascent hydrogen. The nascent hydrogen used in this reaction is obtained by the reaction of hot iron and water vapor, hot zinc and water vapor or zinc diluted HCl.
CHCl3 → CH2Cl2 → CH4Cl → CH4(Methane)
- Electric Cell : E.M.F., Terminal Potential, Internal Resistance
- How Transistor Works : PNP and NPN Transistors
- How p-n Junction Diode works : Forward and Reverse Biasing
- Semiconductors : How Semiconductor works and Types
- X-Rays – Production, Properties, Wavelength and Uses
- Daily use Chemical Compounds and Their Properties
Hydrolysis: When heated with a strong alkali aqueous or alcohol solution, it forms a salt of formic acid. If an aqueous solution of alkali is taken in this reaction, this reaction is called Hydrolysis.
Example: Sodium formate is obtained by heating chloroform with sodium hydroxide solution.
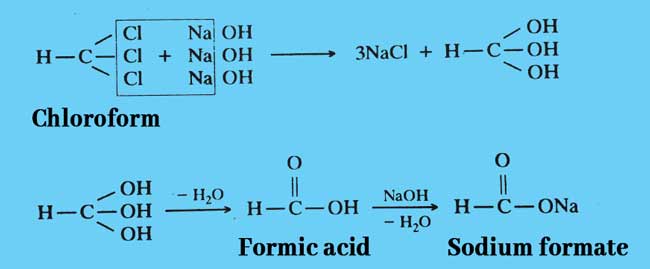
The above reactions can also be written briefly as follows.
CHCl3 + 4NaOH → 3NaCl + NCOONa + 2H2O
Chlorination: The chloroform reacts with chlorine in sunlight to form carbon tetrachloride.
CHCl3 + Cl2 → CCl4 + HCl
Nitration: It reacts with concentrated nitric acid to form chloropicrin. This compound is an insecticide and is used as poison gas in war.
Cl3C – H + NO – NO2 → Cl3C – NO2 + H2O
On heating with silver powder: Acetylene gas is produced by heating chloroform with silver powder.
2CHCl3 + 6Ag → HC ≡ CH + 6AgCl
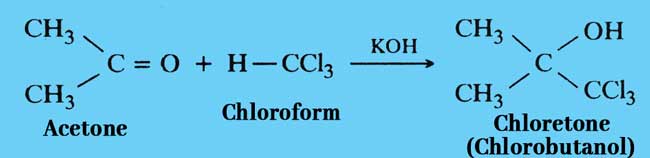
Reaction with Acetone: Chloroform reacts with acetone in the presence of alkali to form chloretone. chloretone is used as a medicine.
Carbylamine Reaction: On heating a primary amine and alcohol caustic potash with chloroform, the extremely deodorant substance isocyanides is formed. This substance is also called carbylamines.
CH3NH2 + CHCl3 + 3KOH → CH3NC + 3KCl + 3H2O
C6H5NH2 + CHCl3 + 3KOH → C6H5NC + 3KCl + 3H2O
This reaction is used to test primary amines.
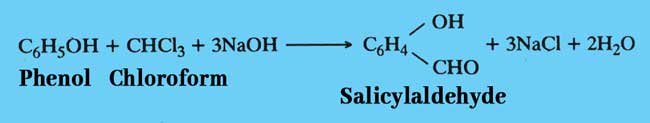
Reimer Thiemann reaction: Salcylaldehyde is formed when chloroform is heated with phenol and a caustic alkali such as NaOH or KOH.
Chloroform Tests
Its smell is sweet and characteristic. It can be identified by its special smell.
When heated with aniline (primary amine) and caustic potash, it forms a highly deodorant carbylamine additive.
Red-brown precipitate is formed due to cuprous oxide(Cu2O) being formed when heated with a fehling solution.
Pure chloroform does not give any precipitate with silver nitrate. The purity of chloroform can be checked by this test. Once purity is checked, it is used as an anesthetic.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
Chloroform Uses
For a few years from now, it was used as an anesthetic
in surgery by adding 30% ether to chloroform.
As a solvent of oil, wax, fat, rubber etc.
In the laboratory as solvent and reagent.
In the manufacture of some useful organic compounds.
Such as chloretone, chloropicrin etc.
in preservation of anatomical specimens
