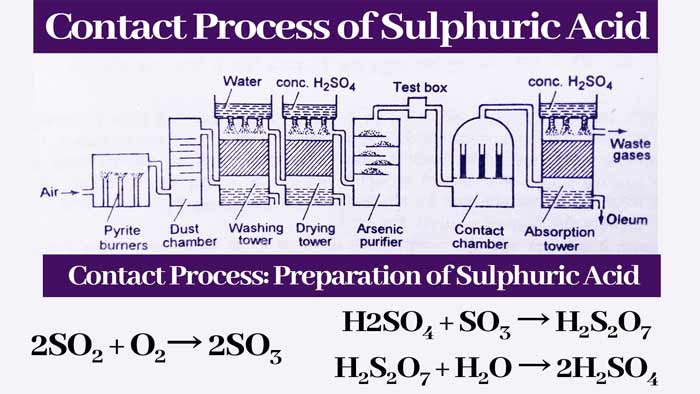
Preparation of Sulphuric Acid by Contact process with Reaction
In this method sulfer di-oxide is oxidized to sulfer trioxide in the presence of platinum containing asbestos or vanadium pentoxide(V2O5). Platinum(Pt) or vanadium pentoxide(V2O5) acts as a catalyst. Platium is a more precious metal, so vanadium pentoxide is used as a catalyst in place of platinum in modern plants. The reaction of formation of Sulfur trioxide(SO3) by the combination of sulfer dioxide(SO2) and oxygen(O2) is exothermic and the number of molecules in it is reduced.
2SO2 + O2 ⇌ 2SO3 + 45,200 cal
According to the law of la Chatelaine-
Higher concentration of reactants helps in higher formation of SO3.
Low temperature helps in the formation of SO3.
High pressure helps in the formation of SO3.
Therefore, in this method the pressure of the gaseous mixture is kept at about 1.5 atmospheric pressure. Low temperature is helpful for more formation of SO3 but optimum temperature for activation of catalyst is 450°C.
Preparation of Sulfuric Acid
The plant used in this method is shown. A brief description of the main parts of this plant and the reactions taking place in it are as follows-
Pyrite Burner:
It is a type of furnace in which iron pyrite (FeS2) or sulfur(s) is burnt in the presence of air. Thus sulfur di oxide gas is obtained.
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
S + O2 → 2SO2
Dust chamber:
The gaseous mixture obtained from Pyrite burner is passed through a chamber where the dust particles present in these gases are removed. These chambers are called dust chambers.
Washing Tower:
The gaseous mixture obtained from the dust chamber is sent to the washing tower. In this tower, water is rained from the top side and the gases received from the dust chamber are flowed from the bottom. The remaining dust particles and some other impurities are absorbed by the water.
Drying tower:
The gases received from the washing tower are passed down to another tower. In this tower, drops of concentrated sulfuric acid keep dripping from the top. The concentrated sulfuric acid absorbs the water vapor present in the gases obtained from the washing tower and the gases obtained from the drying tower are dried.
That is, water vapor is not present in them.
Arsenic Purifier:
The gaseous mixture obtained from the drying tower is sent to the arsenic purifier. Arsenic purifier is filled with ferric hydroxide. It absorbs the impurities of arsenic present in the gaseous mixture obtained from the drying tower and the gases are purified.
Test Box:
In this box the purity of the gaseous mixture obtained from the arsenic purifier is checked. The pure gaseous mixture consists of SO2 gas and the remaining gases of air.
In these boxes, rays of light are passed through the gaseous mixture obtained from the arsenic purifier. If the gaseous mixture is impure, then the particles of the impurities are seen glowing in the test box. A pure gaseous mixture is transparent and no glowing particles are seen in it when light is passed through it.
Contact Chamber or Converter:
The pure gaseous mixture obtained from the test box is sent through iron channels to an iron chamber. This iron chamber is called contact chamber or converter and its temperature is about 450°C. Iron tubes are filled with platinum-containing asbestos or vanadium pentoxide(V2O5). In this cell, SO2 is oxidised to SO3. The major part of the gaseous mixture obtained from the contact chamber is SO3 gas.
2SO2 + O2 → 2SO3
Absorption tower:
The gases received from the contact chamber are sent to the absorption tower. In this tower, the gases are sent from the bottom and the drops of concentrated H2SO4 keep falling from the top.
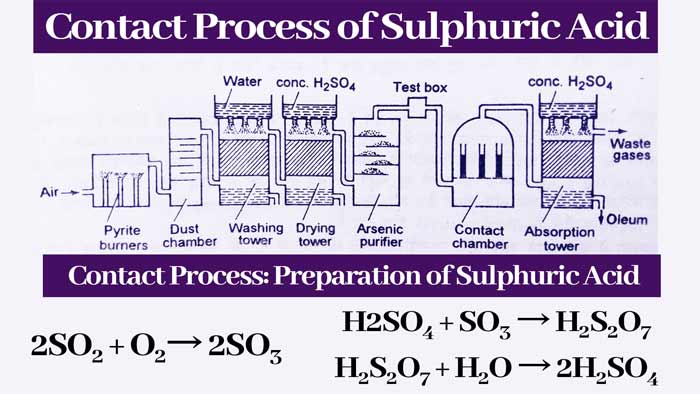
Pieces of quatrz are also filled in this tower. By absorption of concentrated sulfuric acid, sulfur trioxide gas forms fuming sulfuric acid.
H2SO4 + xSO3 → H2SO4.(SO3)x
Concentrated sulfuric acid is obtained by mixing appropriate amount of water in the fuming sulfuric acid obtained from the absorption tower. If x = 1 i.e. the ratio of H2SO4 and SO3 in fuming sulfuric acid is 1 : 1, then –
H2SO4 + SO3 → H2S2O7
H2S2O7 + H2O → 2H2SO4
Comparison of Lead chamber method and Contact method
The similarity between these methods is that in both these methods SO2 is oxidised to SO3 and SO3 is dissolved in water to form sulfuric acid.
In the lead chamber method, SO2 is oxidized to SO3 in the presence of a gaseous catalyst (oxide of nitrogen) and in the contact method, SO2 is oxidized to SO3 in the presence of a solid catalyst (platinum asbastos or vanadium pentaoxide).
Thus, the first reaction is an example of a homogeneous reaction and the second reaction is an example of a heterogeneous reaction.
If platinized asbastos is used as a catalyst in the contact method, then this method is costlier than the lead chamber method. If vanadium pentaoxide is used as a catalyst in the contact method, then this method is cheaper than the lead chamber.
The control of lead chamber method is simple whereas in contact method a lot of care has to be taken. In the contact method, if the impurities of arsenic reach the contact chamber, it acts as a catalytic poison and destroys or reduces the catalytic activity of platinum.
The acid obtained by the lead chamber method is dilute and impure. The acid obtained in the contact method is concentrated and pure.
Physical Properties
Sulfuric acid is oily and colorless liquid. It is soluble in water in all proportions. Adding water to sulfuric acid produces a large amount of heat. Bumping of the acid occurs due to excessive heat and the glass vessel may break or explode.
For this reason, water is never mixed with acid. To dilute the concentrated acid, the concentrated acid is mixed in cold water by stirring it slowly with a glass rod. It is a strong acid. Hence it is also a corrosive material. When it falls on the skin, it puts painful blisters on the skin.
The concentration of sulfuric acid used in the laboratory is 98.5 percent by weight. Its relative density is 1.84 and its normality is 36N. Its boiling point is 338°C. The norlity of dilute sulfuric acid used in the laboratory is 5 N.