Dalton’s law of partial pressure || Numeric Example
Dalton’s law of partial pressure :
When a gas is placed in a vessel, it exerts pressure on the walls of the vessel. If 2 – 3 gases are put together in the vessel, then all these together will exert pressure.
Dalton discovered in 1802, that if two or more gases that do not react chemically with each other are kept in a vessel at a certain temperature, then each gas exerts the same pressure as it does at the same temperature in that vessel. But put it alone. The pressure exerted by a gas present in the mixture alone is called partial pressure.
The total pressure of a mixture of gases is equal to the sum of the partial pressures of its constituent gases. On the basis of this, Dalton presented the following rules related to gases –
When two or more gases that do not react chemically with each other are kept in a vessel at a certain temperature, the pressure of the mixture is equal to the sum of the partial pressures of those gases at the same temperature.
This law is called dalton’s law of partial pressure after its discoverer.
Let there be four different gases A, B, C and D in a mixture of a gas. If their partial pressures are p1 , p2 , p3 and p4 respectively, then the absolute pressure P of a mixture of gases can be expressed by the following equation:
P = p1 + p2 + p3 + p4
Example :- Let us assume that the pressure of a certain amount of air and water vapor in a vessel of 1000 ml volume at 25°C is 95 mm and 25 mm respectively. If the quantities of these two are closed together in the same vessel at the same temperature, then the pressure of the mixture will be 95 + 25 i.e. 120 mm.
This law is used in the volume calculations of gases to find the pressure of dry gas. Often the gases are collected over the water, due to which some water vapor is also mixed with them. The pressure of the moist gas thus obtained is the sum of the pressure of the dry gas and the pressure of the water vapor. The pressure exerted by water vapor at a given temperature is called aquous tension.

Therefore, to find the pressure of the dry gas, the pressure obtained at that temperature is subtracted from the pressure of the gas obtained in the experiment.
Pressure of gas obtained in experiment = pressure of dry gas + vapor pressure
Hence, pressure of dry gas = pressure of gas obtained in experiment + vapor pressure
Example :- The partial pressure of oxygen, hydrogen and nitrogen gas at 25 C is 135, 145 and 225 mm respectively. What will be the total pressure of the mixture of these gases at the same temperature?
Answer :- Oxygen, hydrogen and nitrogen gases do not react with each other at 25°C, so with the help of Dalton’s law of partial pressure
P = p1 + p2 + p3
Whereas P is the absolute pressure of the mixture.
p1 , p2 and p3 are the partial pressures of the constituent gases.
p1 = 135 mm p2 = 145 mm and p3 = 255
so that
P = 135 + 145 + 255
= 535 mm
Hence, the total pressure of the mixture = 535 mm
Example :- Two different gases are filled in two letters. Their pressures are 750 mm and 600 mm respectively. If these two vessels are connected keeping the temperature constant, what will be the pressure of the mixture?
Answer :- Pressure of mixture P = p1 + p2
= 750 + 600
=1350 mm
Pressure of mixture = 1350 mm
Gaseous Diffusion :
In whatever space is available to the gases, they spread evenly. This property of gases is called the property of diffusion and this phenomenon is called diffusion.
The mixing of two or more substances into a homogeneous mixture by itself is called diffusion.
Example :- After placing a jar
full of ammonia gas upside down on a jar filled with air, in some time both the
air and ammonia gases spread equally in both the jars.
Air is heavier than ammonia, but ammonia automatically enters from the lower jar into the upper jar and from the top jar to the lower jar and a homogeneous mixture of both the gases is formed. Similarly, when two cylinders A and B filled with nitrogen and oxygen gases are joined together, both the gases enter each other and spread equally in both the cylinders.
The gases pass through the porous medium. The flow of gases through small pores is also called diffusion. The diffusion rates of gases are determined by diffusion of gases through porous tubes.
The diffusion of light gases (eg – hydrogen) occurs faster than heavy gases (eg – air). This is confirmed by the following experiment –
Uses of Diffusion :
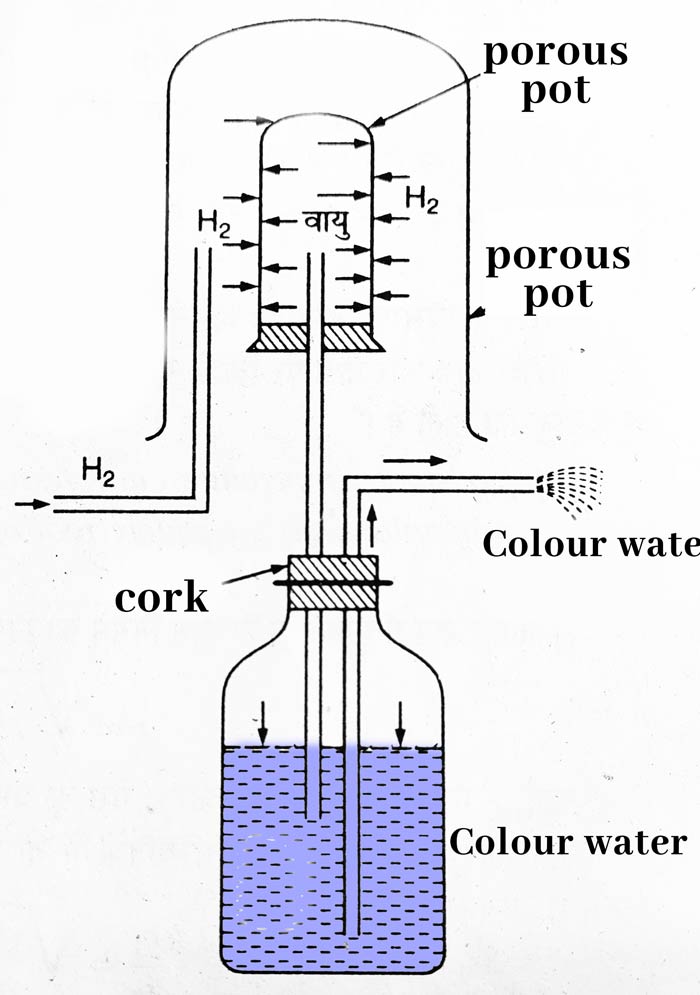
Taking a porous pot, put a cork on its mouth with a glass tube. The other end of the glass tube is attached to a jar filled with colored water. A bent second tube is put in the jar. Make sure that the lower end of both the drains is inside the colored water.

Place a gas jar upside down on top of the porous pot. On passing hydrogen gas in this gas jar, we see that the colored water starts coming out in the form of a fountain with speed. The reason for this is that hydrogen is about 14 times lighter than air, due to which its diffusion occurs quickly.
Therefore the pressure in the porous pot starts increasing. Due to this pressure, the pressure on the colored water also increases and the colored water starts coming out in the form of fountains.
It is clear from the above experiment that the diffusion of light gases occurs faster than heavy gases.
Grahams law of diffusion :-
It has been known from many experiments done on the diffusion of gases that the diffusion rate of a gas depends on its density.
T Graham, 1829, established a relation between the diffusion rate of a gas and its density by experiments, which is called Graham’s law of diffusion.
At constant temperature and pressure the rate of diffusion of a gas is inversely proportional to the square root of the density or molecular weight of gas.
Mathematically, Grahams law of diffusion can be expressed as follows –
r ∞ √ (1 / d)
or r ∞ 1 / √ d
where r is the diffusion rate of the gas and d is the density of the gas.
The relationship between the diffusion rates and their densities of two different gases under the same conditions of temperature and pressure will be –
r1 / r2 = √ (d2 / d1)
where r1 and r2 are the diffusion rates of two different gases whose densities are d1 and d2 respectively.
Density of gas ∞ Molecular mass of gas
so that r1 / r2 = √ (d2 / d1) = √ (M2 / M1)
where M1 and M2 are the molecular weights of two different gases.
Diffusion Rate :-
The volume of a gas that diffuses out in unit time is called the Diffusion Rate of the gas.
Diffusion Rate = Volumn of diffustion gas / Time of diffuses
The ratio of diffusion rates of gases at the same temperature and pressure will be –
r1 / r2 = (V1 / t1) x (t2 / V2) = √ (d2 / d1) = √ (M2 / M1)
It is clear from Grahams law of diffusion that light gas diffuses more quickly than heavy gas.
Application of Grahams law :-
Grahams law of diffusion has the following uses –
In calculating the molecular mass of a gas – if the rates of diffusion of two gases are known and the molecular weight of one is known, then the molecular mass of the other gas can be determined with the help of Grahams law of diffusion –
To find the relative density of gases – If the rates of diffusion of two gases are known, then their relative density can be found with the help of Grahams law of diffusion.
In calculating the diffused amount of gases – If the diffusion speed of gases is known and the time of diffusion is known, then the amount of diffused gases can be found.
In the separation of gases – Due to the different speed of diffusion of gases, they can be separated from the mixture. From the mixture of hydrogen and air, hydrogen is quickly separated by diffusion.
In removing the odour – the cause of the foul smell and toxic gases getting diffused in the air keeps getting removed.
As a marsh gas indicator – diffusion rule is used in practical application to obtain information about the presence of explosive marsh gas in coalmines. The equipment used for this is called marsh gas indicator.
Marsh gas (methane) being lighter than air, gets diffused quickly in the poseurt pot. Due to which the pressure starts increasing in the A part of the U tube and the temperature of the mercury starts increasing in the B part.
The mercury rises up and touches the wire attached to the electric bell. The electric bell starts ringing as soon as it makes contact with the electric wire. In this way, the workers working in the mines are immediately alerted of toxic gases with the help of this indicator.
