Isotopes of Hydrogen: Deuterium
Isotopes of Hydrogen:
- Protium
- Deuterium
- Tritium
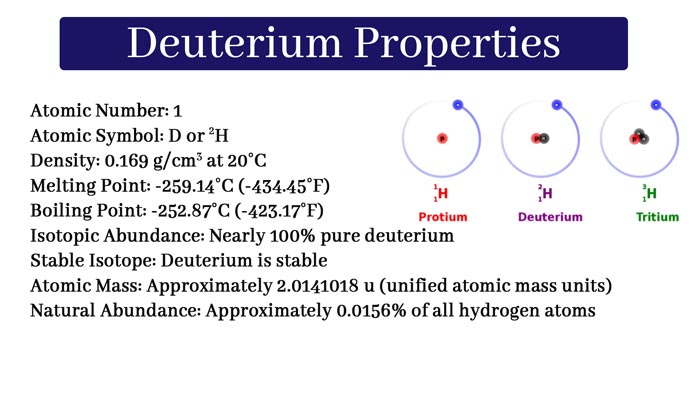
Three isotopes of hydrogen are known. Their atomic number is 1 and their mass numbers are 1, 2, and 3 respectively. These institutes are called Hydrogen-1(protium), Hydrogen-2(deuterium) and Hydrogen-3(tritium).
Their symbols are 1H1, 1H2, 1H3 respectively. In chemical equations, they represent H, D, and T respectively. Each of them has one electron and one proton and the number of neutrons is 0, 1, 2 respectively.

Protium is also known as light hydrogen and deuterium as heavy hydrogen. All the three sites of hydrogen, only the protium and deuterium are stable. And tritium is temporary. It is a radioactive isotope of hydrogen. Its half-life is 12.32 years.
Ordinary hydrogen contains 99.98% protium, 0.02% deuterium and an almost negligible amount of tritium. Hence, about 1000 parts of ordinary hydrogen contain about 1 part of heavy hydrogen.
Deuterium:
Like hydrogen, deuterium has two atoms in one Atom. Hence, it is a double standard gas. Harold Urey was discovered in 1931. for the discovery of Deuterium, Harold Urey received the Nobel Prize in 1934.
Deuterium Preparation:
Heavy Water:
The oxide (D2O) of Deuterium is also called heavy water. The main method of obtaining deuterium to heavy water is the following:
1) Deuterium is obtained by the action of sodium from heavy water. Deuterium gas is obtained.
2Na + 2D2O → 2NaOD + D2
2) The electrical decomposition of heavy water also gives duet gas. For the decomposition of heavy water, small amounts of phosphorus pentaoxide or sodium carbonate are added to it. And uses the electrode of nickel.
2D2O → 2D2 + O2
3) Duet gas is also obtained when heavy water vapor flows over a blood-heated iron, zinc, or magnesium metal.
2Fe + 4D2O → Fe3O4 + 4D2
Zn + D2O → ZnO + D2
Mg + D2O → MgO + D2
The above reactions are used both for making small amounts of duet gas in the laboratory and for its industrial manufacture.
Hydrogen
About 6,000 parts of ordinary hydrogen contain about 1 part deuterium. The following is the main method of separating duet from ordinary hydrogen.
Diffusion Method:
According to Graham’s law of diffusion, the diffusion speed of a gas is inversely the square root of its atomic mass.
r ∞ 1/√M
Therefore, light gases expand faster than heavy gases. By expanding ordinary hydrogen into a porous pot, light hydrogen will expand rapidly. Thus duet gas can be obtained.
Fractional Distillation Method:
In this method, liquid hydrogen is effectively distilled in a vacuum. Light hydrogen (boiling point = 20.28 K) evaporates first and the Deuterium (boiling point = 23.59 K) remains in the distillation flask. This method was first used by Harold Urey in 1931.
Physical properties:
It is a colorless, odorless and tasteless gas. It is insoluble in water. Its weight and density are two times the weight and density of hydrogen. Its boiling point is 23.59 K.
Chemical Properties:
The chemical properties of isotopes are similar. Hence, Deuterium and hydrogen also have similar properties. The speed of chemical reactions of Deuterium and hydrogen varies. The chemical reactions of hydrogen are about twice as fast as the chemical reactions of Deuterium. The following are some of the key chemical reactions of It.
Burning:
It forms heavy water burning with air.
2D2 + O2 → 2D2O
Halogen:
Duetro-Halides is formed by acting with halogens in appropriate positions.
D2 + F2 – Low temperature/dark → 2DF
D2 + Cl2 – Sunlight → 2DCl
D2 + Br2 -4000C→ 2DBr
D2 + I2 – Pt → 2DI
Nitrogen:-
3D2 + N2 –Fe+Mo → 2ND3 (Deutero-ammonia)
Sulfur:
D2 + S –high temperature → D2S (deuterium sulfide)
Metals:
2Na + D2 – 3500C → 2NaD (Sodium deuteride)
Ca + D2 – 4000C → CaD2 (Calcium deuteride)
Summative reactions:
It exhibits Summative reactions with unsaturated hydrocarbons.
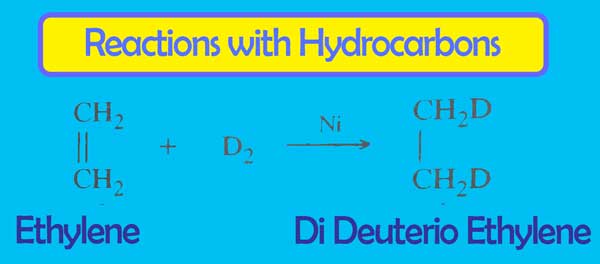
Exchange Reactions:
In suitable conditions, it substitutes hydrogen from compounds of hydrogen by deuterium. Such reactions are called Exchange Reactions.

Uses of Deuterium:
It is used as a projectile in nuclear reactions and as a reactor in nuclear fusion processes.
It is used in making compounds of deuterium.
It is used as a tracer element in the study of the processes of various reactions and processes.
Used in making heavy water which is used for moderator in nuclear fission processes.