Electric Cell, Terminal Potential difference, EMF and Internal Resistance
What is an electric cell
An electric cell is a device that converts chemical energy into electrical energy and maintains a constant flow of charge in a circuit. It consists of two rods of different metals called electrodes or plates.
It is immersed in a liquid called electrolyte. This liquid has this property that when the plates are immersed in it, one plate becomes positively charged and the other becomes negatively charged.
When both the plates are connected with a wire, the charge starts flowing in the wire. Such a chemical reaction takes place in the electrolyte inside the cell, due to which the charges on the plates keep on filling and the charge flow in the wire remains.
In this way the cell keeps on converting chemical energy into electrical energy.
E. M. F. of a Cell :-
To maintain the continuous flow of charge in an electric circuit, some work has to be done. This work is done by the cell. The energy that is liberated in the chemical reactions taking place in the cell is what drives the charge in the circuit.
In this way the cell converts the chemical energy of its electrodes and electrolytes into electrical energy.
The energy given by the cell in moving a unit charge through the whole circuit is called Electromotive force (EMF) of the cell.
EMF is a characteristic of each cell, which depends on the nature of the plates and electrolytes used in the cell. The amount of electrolyte and the size of the plates or the distance between them does not affect it.

If the energy given by the cell in passing a q coulomb charge in a circuit is W joul, then the EMF of the cell
E = W / q joule / coulomb
The unit of EMF is joule / coulomb which is called Volt. If the energy given by the cell on passing 1 coulomb of charge in a circuit is 1 joule, then the EMF of the cell is 1 Volt.
The direction of the current sent by the cell in the circuit is from the ‘inside of the cell’ negative electrode to the positive electrode.
In other words, the positive charge inside the cell flows from the negative electrode to the higher potential. Therefore, the EMF of the cell is directed from the negative electrode to the positive electrode inside the cell.
Terminal Potential difference :
Let the EMF of a cell (E) connected in an electric circuit and (W) is the energy given by the cell when (q) charge flows through the circuit. Then
E = W / q
The charge q would be the same in all parts of the circuit. If the energies spent in different parts of the circuit are W1, W2, W3, ….. then their sum will always be W. Therefore
E = W / q = (W1 + W2 + W3 + …..) / q = W1 / q + W2 / q + W3 / q + ……
It is clear that V1, V2, V3 are the energies spent in different parts of the circuit in carrying a unit charge. They are called terminal potential difference of different parts of the circuit.
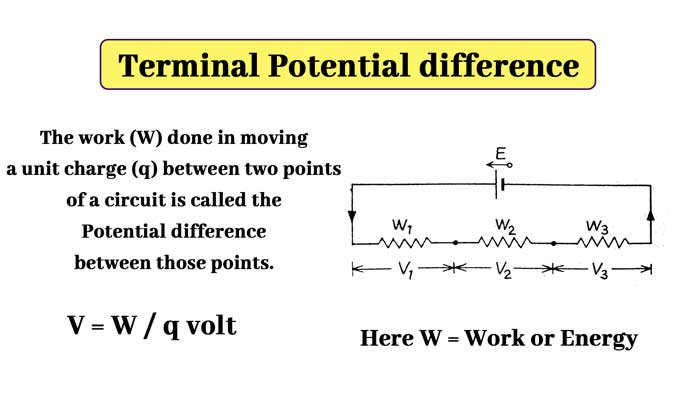
Thus, if W’ joule of work is to be done (W’ joule is the work expended) on passing a q coulmn charge between two points of a valid circuit, then the terminal potential difference between those points will be V = W’ / q volt. The potential difference is measured with a voltmeter.
Internal Resistance of a Cell :
When we connect a cell with a wire, then the electronic current in the wire flows from the positive plate of the cell to the negative plate, and in its solution inside the cell, from the negative plate to the positive plate.
Just as the wire exerts resistance in the path of electric current, similarly the solution of the cell also imposes resistance in the path of the electric current. This resistance is called the internal resistance of the cell.
Internal Resistance of Cell depends on the following factors.
· It is directly proportional to the distance between the two plates of the cell,
· Inversely proportional to the area of the plates dipped in solution
· Increases as the concentration of electrolyte increases.
The internal resistance of the cell does not remain constant, but gradually increases when the cell is used.
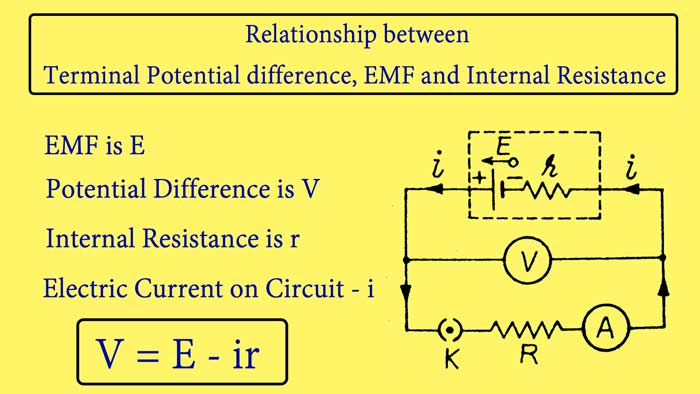
Relationship between Terminal Potential difference, EMF and Internal Resistance :-
A cell whose EMF is E and internal resistance r is connected to a resistance wire R and A Ameter by a key K. A voltmeter V is connected between the plates of the cell. The internal resistance of the cell can be understood to be added in series with the r cell. On closing the key K, the cell starts sending a electric current in the circuit, whose value is read from the ameter.

Let this value be i and the text of the voltmeter is V.
Let the current i, flow in the circuit for time t. If q is the charge flowing in the circuit in time t, then the energy given by the cell.
W = Eq
W = Eit
Outside the cell, there is a potential difference V between the ends of the resistance R. So the work done outside the cell –
Wout = Vit
The internal resistance of the cell is r. Therefore, when the current i flows in the circuit, the potential drop in the electrolyte inside the cell will be v = ir. So the work done inside the cell –
Win = vit = irt
W = Win + Wout
Eit = Vit + i2rt
E = V + ir
V = E – ir
But V is also the terminal potential difference available between the plates of the cell. So it is clear that when the cell is giving current then the terminal potential difference V between its plates is less than its EMF E. The reason for this is because of internal resistance in the cell, due to which the potential drop within the cell becomes ir.
It is clear from the above equation that the higher the value of i, the lesser the value of V will be. This means that the more current we take from the cell, the less will be the potential difference between its plates.
Thus, EMF E is its own characteristic of each cell and its value remains constant for a cell, whereas the value of terminal potential difference (V) decreases as more and more current is taken from the cell.
If i = 0 in the above equation then
V = E ;
When current is not being drawn from the cell, then the terminal potential difference between the plates of the cell is equal to the EMF of the cell. Therefore, when the key is open, the value of EMF E of the cell is obtained from the text of the voltmeter.
If the current in the circuit is i, then the potential difference between the ends of the resistance R –
V = iR
iR = E – ir
E = iR + ir = i (R + r)
i = E / (R + r)
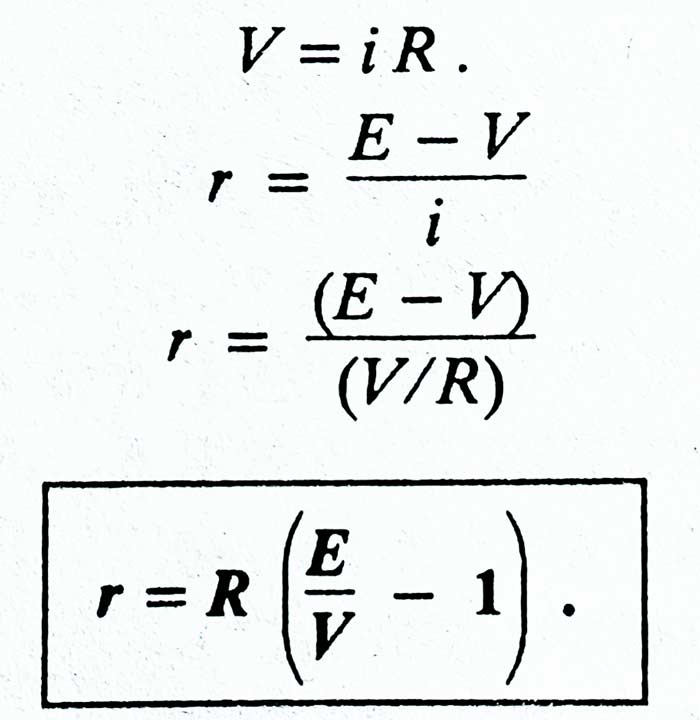
Here (R + r) is some resistance of the circuit. Therefore, it is clear that the value of the current drawn from the cell is obtained by the ratio of the EMF of the cell and the total resistance of the circuit.
If there is more than one cell in the circuit, then the value of the current is obtained by the ratio of the net emf in the circuit and the total resistance of the circuit. This statement or the above equation expresses the law of conservation of energy for a valid circuit.
When current is drawn from the cell, the direction of current inside the cell is from the negative plate of the cell to the positive plate and the terminal potential difference V between the plates of the cell is less than the EMF E of the cell.
V = E – ir
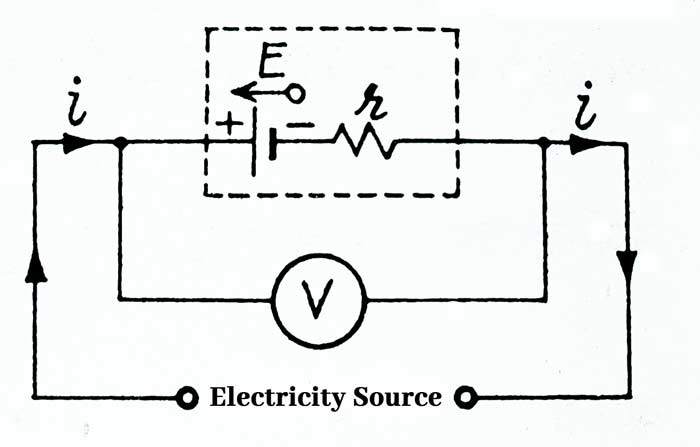
If we send a current from the cell to any other valid source to charge a cell, then the direction of current inside the cell will be from positive plate to negative plate. In this case the terminal potential difference V between the plates of the cell will be more than the Emf E of the cell.
V = E + ir