Electrochemistry : Electrolytic cell, Mechanism and Cell Structure
When electricity causes a chemical reaction, then this process is called electrochemistry. There is a definite relationship between electrical energy and chemical energy.
The branch of chemistry in which the relationship between electrical energy and chemical energy and their transformation into each other is studied is called electrochemistry.
Under electrochemistry, those chemical reactions are studied in which electrical energy is liberated or absorbed, these reactions can be of two types –
Electrolysis
Electro – Chemical Cell
Electrolysis :-
When an electric current is passed through the molten state of certain substances or their solutions, the substance decomposes into its constituent parts.
Example: By passing an electric current through water in the presence of dilute acid, water decomposes into its constituents oxygen (O2) and hydrogne (H2).
2H2O → 2H2 + O2
Such substances which get decomposed into their components by passing current are called Electrolytes and this process is called electrolysis. Usually acids, bases and salts are electrolytes.
Electrolytes are made up of electrically charged particles called ions. There are two types of Ions, which have positive electric charge, they are called cations and those which have negative electric charge are called anions.
Daily use Chemical Compounds and Their Properties
Solar Energy : Light Waves, Reactions and Uses
Bohr-Bury Scheme | Bohr and Bury Rule | Chemistry Notes
Similarly, electric current cannot flow in solid sodium chloride, so solid sodium chloride is first brought to the molten state. For this, a heater is used inside the decomposition cell. When sodium chloride is molten, when electric current is passed through it, due to the legal decomposition of sodium chloride, sodium is obtained at the cathode and chlorine gas is obtained at the anode.
2NaCl + Energy (Electric Current) → 2Na + Cl2
Therefore, electrolytes are the reactions in which a molten or aqueous solution of a compound is chemically decomposed by the effect of electric current.
A special device is used for electrolytes, which is called an electrolytic cell.
In an electrolytic cell, molten sodium chloride breaks down into sodium ion (Na+) and chloride ion (Cl–).
NaCl → Na+ + Cl–
The positively charged ions (Na+) are attracted to the negatively charged electrode cathode and get converted into sodium atom after gaining one electron.
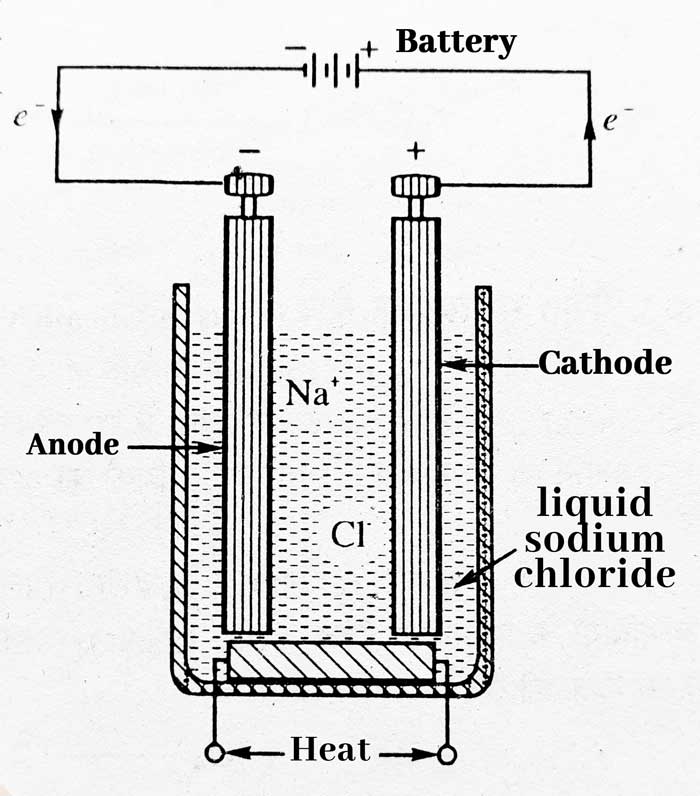
at the cathode
Na+ + e– → Na (reduction)
The acquisition of electrons is called reduction. Here Na+ is reduced to Na. The negative ions (Cl–) are attracted to the positively charged electrode anode and change into a chlorine atom by removing an electron. Two chlorine atoms combine to form a chlorine molecule (gas).
The loss of electron is called oxidation. Here Cl– oxidation takes place in Cl.
at the anode
Cl– → Cl + e– (oxidation)
Thus molten sodium chloride decomposes into sodium metal and chlorine gas.
Cl + Cl → Cl2 (g)
In fact, sodium metal and chlorine gas are produced in the industry by this method. The complete process of electrolysis of molten sodium chloride can be written as follows –
Na+ + Cl– → Na(s) + 1/2Cl2 (g)
In fact, sodium metal and chlorine gas are produced in the industry by this method. The complete process of electrolysis of molten sodium chloride can be written as follows –
Electro Chemical Cell :
In electrolysis reaction, electric current produces a chemical reaction, on the contrary, electric current can also be generated by chemical reaction. Electrical energy is produced by many displacement reactions of metallic compounds.
When a zinc rod is placed in an aqueous solution of copper sulfate (CuSO4), zinc sulfate (ZnSO4) is formed as a result of the reaction and goes into the solution and gets deposited on the copper-zinc rod.
Zn + CuSO4 → ZnSO4 + Cu
In the above reaction, zinc displaces copper in aqueous solution of copper sulfate, releasing it as a metal. This reaction takes place on taking copper sulfate solution of appropriate concentration. This fact was first discovered by itli scientist Luigi Galvani. The device in which electricity was generated by the appropriate reaction was called electrochemical cell.
Therefore, electrochemical cell is a device which is used to generate electrical energy from spontaneous chemical reactions.
That is, an electrochemical cell is a device that converts chemical energy into electrical energy.
Another scientist Volta, a contemporary of scientist Galvani, also independently discovered the electrochemical cell. In honor of these two scientists, this cell is also called Galvani cell or Voltaic cell.
Simple Cell or Voltaic Cell :
Simple voltaic cell works on instantaneous oxidation – reduction reaction. In this, electrons from the substance being oxidised go to the substance to be reduced.
Example: – In a simple voltaic cell, zinc (Zn) metal is oxidized by copper ion (Cu++) to generate electric current.

Cell Structure :- It has the shape of glass. In which dilute sulfuric acid (H2SO4) is filled as electrolytes. A copper rod is attached to this vessel. These are called electrodes. Connecting screws are attached at the ends of these rods.
When a tourch bulb is attached by a wire between the ends of these two rods, the bulb starts burning. It is clear from this that electric current is flowing in the wire.
Mechanism :– According to the ionic theory of liquids, sulfuric acid (H2SO4) always contains hydrogen ion (H+) and sulfate ion (SO4–). Which have positive and negative charges respectively –
According to the free electron theory of metals, copper and zinc rods always have some free electrons. Due to which there are some copper ions (Cu++) in the copper rod and some zinc ions (Zn++) in the zinc rod.
When copper and zinc rods are immersed in sulfuric acid, some hydrogen ions (H+) of this acid reach the copper rod and some ions (Zn+) from the zinc rod come into the acid. Some free electrons of copper combine with hydrogen ions (H+) to form hydrogen atoms and hydrogen atoms combine to form H2.
H+ + e– → H
H + H → H2
This leads to reduction of electrons on the copper rod i.e. copper rod becomes positively charged, hence it is called anode. Thus its potential becomes + 0.46 volt higher than that of acid. Similarly, some zinc ions (Zn++) from the zinc rod join with the sulfate ions of the acid to form zinc sulfate.
Zn++ + SO4— → ZnSO4
This leads to an excess of electrons on the zinc rod, that is, the zinc rod becomes negatively charged, hence it is called a cathode. Its potential becomes 0.62 volt less than that of acid.
That is, its potential is – 0.62 volt. Thus the potential difference 0.46 – (-0.62) = 1.08 volts between these two rods is called the electric carrying force of the cell. Copper rod is called positive electrode and zinc rod is also called negative electrode.
When copper and zinc rods are given by wire, electrons start moving from the zinc rod (due to excess of electrons) to the copper rod (due to lack of electrons).
Due to which the negative charge of the zinc rod and the positive charge of the copper rod starts decreasing. To make up for the lack of positive charge on the zinc rod, SO4– ion from the acid enters the zinc rod and H + ions from the acid start reaching the copper rod to make up for the lack of positive charge on the copper rod.
H+ ion converts into hydrogen atom by giving its positive charge to the copper rod and H2 gas starts coming out in the form of bubbles. The sulfate ion (SO4) reacts with zinc by giving a negative charge to the zinc rod to form zinc sulfate.
Zn + SO4 → ZnSO4
H+ ion converts into hydrogen atom by giving its positive charge to the copper rod and H2 gas starts coming out in the form of bubbles. The sulfate ion (SO4) reacts with zinc by giving a negative charge to the zinc rod to form zinc sulfate.
Zn + H2SO4 → ZnSO4 + H2
Therefore, dissolving zinc in acid is the only source of electrical energy.
Dry Cell :
The cells used in Radio Tourch trangister toys etc. are dry cells. The credit for the invention of the dry cell goes to G Leclanche in 1868.
Dry Cell Structure:
The dry cell consists of a vessel of zinc (Zn) in the center of which is a mixture of manganese dioxide (MnO2) and carbon powder around a rod of carbon. Ammonia chloride (NH4Cl) is present in the remaining part of the vessel.

In this cell, the carbon rod acts as the anode and the zinc metal vessel acts as the cathode. The paste of ammonia chloride acts as an electrolyte.
The dry cell does not dry completely. It also contains some water which helps in the movement of ions. The upper part of the cell is closed with lacquer or leather, due to which the water vapor present inside the cell is saved from evaporation. A brass cap is placed over the carbon rod and the sides of the cell are covered with thick paper or neck.
Working of dry cell : The mechanism of dry cell is based on instantaneous oxidation reduction reaction. In this, the transfer of electrons from the zinc vessel to manganese dioxide takes place through the external circuit.
When the external circuit of a dry cell is connected with a wire between a rod of carbon (graphite) and a vessel made of zinc, the zinc atom (Zn) gets oxidized to zinc ion (Zn++) and electrons are liberated.
Zn → Zn++ + 2e– (oxidation)
The electrons thus generated begin to flow through the wire towards the carbon rod and generate electric current.
When these electrons approach the carbon, they reduce manganese dioxide (MnO2) to manganese trioxide (Mn2O3) in the presence of ammonia ion (NH4+) of ammonia chloride (NH4Cl).
2MnO2 + 2NH4+ + 2e– → Mn2O3 + 2NH3 + H2O
As long as zinc is oxidized and manganese dioxide (MnO2) is reduced, the current flows through the cell’s external circuit.
Uses: – The biggest advantage of dry cell is that due to the absence of liquid in it, it is light and it can be easily carried here. Many types of dry cells like silver cell and lithium cell have been made. Which are used in calculators, watches and cameras.
Defect: – It cannot be charged again when the power is stopped in it, which is one of its drawbacks. This is because the reactions taking place in it are not reversible.
Storage Cell :
Generally, after using electrochemical cells once, they cannot be used again, that is, the chemical reactions that take place in the cell cannot be reversed by passing an electric current. But some such cells are also used now, which can be used again after being used once, such cells are called storage cells.

Lead storage cell or battery is most suitable among the accumulator cells in general use. It is used for driving car, vehicle etc and for many domestic works.
Therefore, the cells that are re-charged are called storage cells. These types of cells have two lead electrodes. One of which is covered with lead oxide on the electrode. Dilute sulfuric acid is used as an electrolyte.
When the cell is used, lead gets deposited on the lead oxide electrode and electric current is generated and when electric current is passed through the cell, lead and sulfuric acid are produced again as a result of the opposite reaction. This reaction is reversible, which takes place as follows.
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
This is the reason that storage cells can be charged again and again, thus they can be used again and again.
Structure of Lead Storage Cell: – Lead storage cell is a vessel made of hard rubber, in which there are positive charge plates made of lead paroxide (PbO2). All positive charge plates are connected to a terminal, which is called positive charge terminal. Negative charge plates made of spangi lead are attached, which is connected to the negative charge terminal of the storage cell.
In the storage cell, positive charge plates and negative charge plates are arranged in an alternate order, that is, a positive charge plate is followed by a negative charge plate and a negative charge plate is followed by a positive charge plate.
Separating plates of porous wood, glass or rubber are kept between each positive charge plate and negative charge plate. Dilute sulfuric acid (H2SO4) acts as the storage cell’s electrolyte. Lead acid cell is represented as follows –
O– [Pb | PbSO4(s)]
O– [H2SO4(40%) | PbO2(s)]
O+ [Pb(s)]
Working of Lead Storage Cell – The mechanism of the lead storage cell is based on an instantaneous oxidation reduction reaction, in which electrons are transferred from the “lead anode” to the ‘lead dioxide’ cathode by an external circuit.
The following reactions take place when a storage cell is used –
(i) cathode
H2SO4 → 2H+ + SO4—
PbO2(s) + 2H+ + 2e– + H2SO4 → PbSO4(s) + 2H2O
(ii) Anode
Pb + SO4— → PbSO4 + 2e–
Therefore, during use, both the electrodes of the cell are covered with a layer of lead sulfate and the relative density of the electrolyte decreases to 1: 1 and the voltage of the battery decreases. In this case the battery is declared disabled.
Recharging of Battery :- At the time of recharging the lead storage cell, by taking some resistance in the middle, connect the anode of the immersed cell to the main wire. That is, the electrode which acts as a cathode, acts as an anode when charged. During this the following reaction takes place –
Cathode :
PbSO4 (s) + 2H+ + 2e– → Pb (s) + H2SO4
Anode :
PbSO4 (s) + SO4— + 2H2O → PbO2 (s) + 2H2SO4 + 2e–
Complete Reaction :
2PbSO4 (s) + 2H2O (l) → Pb (s) + PbO2 + 4H+ (aq) + 2SO4—(aq)
Once charged, the positive plate becomes lead paroxide (PbO2) and the negative plate becomes spongy lead (Pb), meaning the chemical reactions in this cell are variable. After charging the storage cell, the cell is used again as a source of current.
The voltage of the storage cell depends on the number of minus and plus plates present in it.
A cell provides a potential of 2 volts. Generally some cells of 6 or 12 volt are stored.
Some other storage cells are nickel-iron and nickel-cadmium storage cells, which are used in aircrafts.
