The electron configuration of Elements || How do you do an easy electron configuration?
Electron Configuration: Aufbau is a word of german language. It is not a scientist’s name. It means building up.
The electrons in the subshell of atoms fill according to the Aufbau principle.
Aufbau Principle
Electrons enter the subshells of atoms in the increasing order of energy.
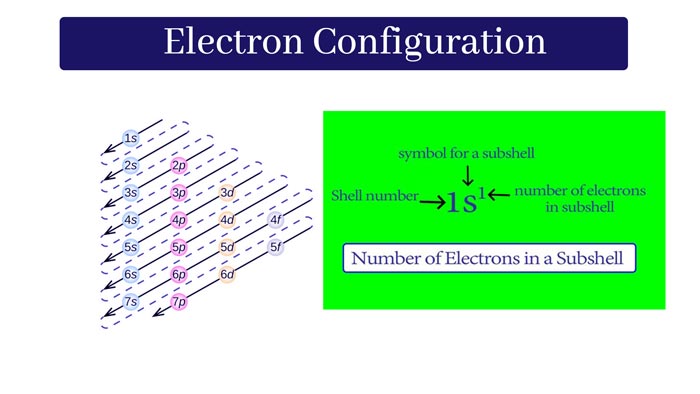
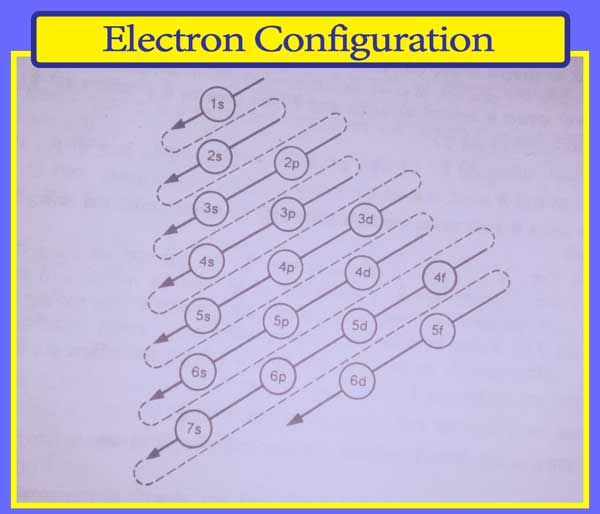
The following is the increasing order of energies of the subshells of atoms:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
The number before s, p, d, and f word is the main quantum number of electrons of its subshells.
It is also called the serial number of shells or shells of atoms.
This is the easy way of remembering the increasing order of energies of the atoms’ subshells.
The energy of the 1s subshell is the lowest among all the subshells, it means the stability of the 1s subshell is the highest and the electron will first enter into this subshell.
The maximum number of electrons in s, p, d, and f subshells can be 2, 6, 10 and 14 respectively.
So after 2 electrons in a 1s subshell, electrons enter the 2s subshell. After fill, 2s subshell electrons enter into 2p subshell. This sequence continues until all the electrons of the atom enter the subshell.
Electron Configuration
The arrangement of an electron in the subshell of an element’s atoms is called the electron configuration of that element. To demonstrate the management of electrons in the subshells of atoms of an element C, P, D, F uses the notation.
A number of electrons in a subshell is demonstrating as a given example:
For example Electronic configuration of Nitrogen (Atomic number = 7) is 1s2, 2s2, 2p3. It means that its first subshell of a shell filled with 2, second s subshell of a shell filled with 2 and 2nd p subshell of shell filled with 3 electrons.

The actual electronic configuration of the elements is done by a detailed mathematical solution of their chemical properties, spectrum properties, and the Schrodinger wave equation.
There is a simple difference in the electronic configuration of some elements and the electronic configuration based on their Aufbau principle. These electronic configurations are exceptions of the Aufbau principle.
Electronic structures with half-filled and full-filled orbitals are generally more stable. On this basis, the difference between the actual electronic configuration of Cr and Cu and the electronic configuration written based on the Aufbau principle can be explained.
Chromium Cr (atomic number = 24) based on the Aufbau principle that the 3d subshell must have 4 electrons. If an electron moves from a 4s subshell to a 3d subshell, then there will be 5 electrons in the 3d subshell that is, 3d subshell will be half-filled.
So chromium(Cr) has 5 electrons in its 3d subshell.
Electron configuration of Chromium as Aufbau principal:
1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d4
Actual Electron configuration of Chromium:
1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d5
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
Elements are divided into four blocks based on their electronic configuration, these are called elements of C, P, D and F blocks respectively. The last electron in the elements of the S-block, the last electron in the sub-particle, the last electron in the elements of the P-block, the last electron in the D-block, the electron in the D-sub-particle, and the last electron in the elements of the F-block gets entry into.
In the process of ionization of elements, the normal final subshell electrons are discarded. But in the ionization of the elements of D-block, the electrons of the s-subshell are first discarded and after that, the electrons of the d-subshell are discarded if necessary.
Example:
Electron configuration of Fe (atom number = 26) , Fe2+, and Fe3+
Fe : 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d6
Fe2+ : 1s2, 2s2, 2p6, 3s2, 3p6, 3d6
Fe3+ : 1s2, 2s2, 2p6, 3s2, 3p6, 3d5
To write the electron configuration of the elements, the subshell is often written in the order of electrons filling them, but the subshell is also written in the order of the shells.
In this Fe example, we can write given two type
Fe : 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d6
Or
Fe : 1s2, 2s2, 2p6, 3s2, 3p6, 3d6, 4s2
Electron configurations of elements are also sometimes written in brief form. Only the number of electrons is displayed in the shells in brief form.
Fe : 2, 8, 14, 2
Klechkowski Rule
With the help of the Aufbau Principle to remember the order after the electrons enter the subshell of the atoms. Aufbau Principle is a German language word. Aufbau Principle is the gift of German scientists. The order of the entry of electrons into the subshell of atoms can also be determined with the help of two rules given by the Russian scientist Kalechkowski.
Klechkowski First Rule or n + l rule:
According to this rule, the electron enters the subshell first, for which the value of n + l is low.
For example n = 1 and l = 0 for 1s subshell. And n = 2 and l = 0 for 2s subshell. So that n + l = 1 for 1s and n + l = 2 for 2s subshell. Therefor electron will enter 1s first then 2s.
Klechkowski Second Rule:
If n + l is equal for two subshells, electrons enter the first of these two subshells which the value of n is low.
Example:
N + l = 3 + 2 = 5 for 3d subshell and N + l = 4 + 1 = 5 for 4d. they are both equal. So value of n is low for 3d subshell and value of n is high for 4d. therefore electron will enter in 3d subshell first.
Summary :
The electron configuration of an atom describes the
distribution of its electrons among the various atomic orbitals. It's typically
written in a notation that indicates the energy levels (shells) and subshells
(orbitals) occupied by electrons. Here's how electron configuration notation
works:
Principal Energy Levels (Shells): These are represented by
the numbers 1, 2, 3, 4, etc. They indicate the main energy levels or shells of
an atom.
Subshells (Orbitals): Within each principal energy level,
there are subshells, which are represented by the letters s, p, d, and f. Each
subshell has a specific shape and can hold a certain number of electrons:
s subshell: Can hold up to 2 electrons, spherical in shape.
p subshell: Can hold up to 6 electrons, composed of 3
dumbbell-shaped orbitals.
d subshell: Can hold up to 10 electrons, composed of 5
orbitals with complex shapes.
f subshell: Can hold up to 14 electrons, composed of 7
orbitals with even more complex shapes.
Electron Spin: Each electron in an orbital has a unique
quantum number called spin, which can have one of two values: +1/2 or -1/2.
Aufbau Principle: Electrons fill atomic orbitals in order of
increasing energy levels, following the sequence 1s, 2s, 2p, 3s, 3p, 4s, 3d,
4p, 5s, etc.
Pauli Exclusion Principle: No two electrons in the same atom
can have the same set of four quantum numbers. Therefore, an orbital can hold a
maximum of two electrons with opposite spins.
Hund's Rule: When filling degenerate orbitals (orbitals of
the same energy), electrons occupy empty orbitals singly before pairing up, and
all electrons in singly occupied orbitals have the same spin.
For example, let's consider the electron configuration of
carbon (C), which has an atomic number of 6:
1s2 2s2 2p2
This notation indicates that carbon has six electrons
distributed as follows:
2 electrons in the 1s orbital (the first shell).
2 electrons in the 2s orbital (the second shell).
2 electrons in the 2p orbital (the second shell), with one
electron in each of the three available 2p orbitals.
This notation succinctly represents the arrangement of
electrons in an atom. Let me know if you need the electron configuration for a
specific element!