Electron: Discovery, Charge and mass of Electron
Fundamental Particles of the Atom
According to Dalton’s atomic theory
until about 100 years ago, atoms were considered indivisible but now it
has been proved that atom is divisible and consists of electrons,
protons, and neutrons.
Therefore, the fundamental particles of an element do not have its atoms.
But the fundamental particles in all substances are
- Electrons
- Protons
- Neutrons
In chemical reactions, an atom participates in an almost indivisible form and is not destroyed in its form.
The same type of atoms has the same number of electrons, protons and neutrons. Electrons in different types of atoms have a difference in the number of protons and neutrons.
Often some other particles such as positron, neutrino, antineutrino and mason are also obtained when the atom is split.
The importance of these particles in the atomic structure is very low because these particles are temporary.
The main characteristics of electron, proton and neutron are given below.
| Particles | Symbol | Mass(a.m.u.) | Mass | Charge | Discovered By |
|---|---|---|---|---|---|
| Electron | e, ₋₁e⁰ | 0.00055 | 9.1095 x 10⁻²⁸ | -1 | J. J. Thomson(1897) |
| Proton | p, ₁H¹ | 1.00728 | 1.6726 x 10⁻²⁴ | +1 | Rutherford(1919) |
| Neutron | n, ₀n¹ | 1.00867 | 1.6750 x 10⁻²⁴ | 0 | Chadwick(1932) |
The mass in grams in the atomic particles of the atom makes it clear that electron, proton and neutron are superfluous particles. Gram is not a suitable unit for the mass of such small particles.
The unit corresponding to the mass of these particles is the atomic mass unit (emu) or (u).
The relative mass of the original particles in the atom shows that the mass of proton and neutron are approximately equal and the mass of electron is negligible compared to the mass of proton and neutron.
The charges of electron and proton are similar in magnitude (-1) and opposite in nature. The charge of electron is -1.603 x 10-19 coulomb and the charge of proton is + 1.603 x 10-19 coulomb. Since the low charge is not found on any particle, hence their charge is called unit negative and unit positive respectively.
Discovery of Electron
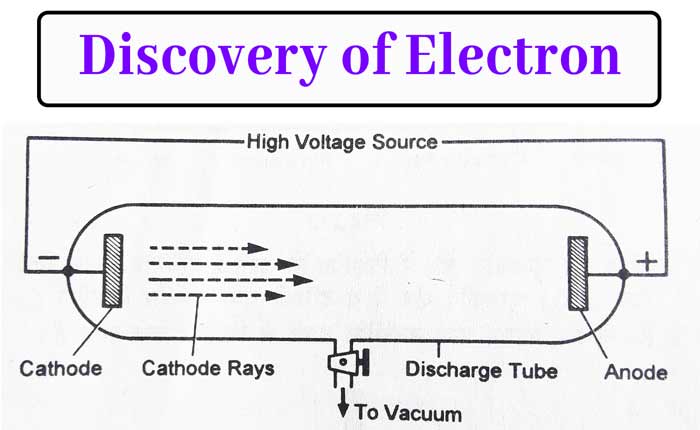
In 1859, Julius Plucker flowed current in gases at low pressure. In a glass tube of about 60 cm long, a gas was taken at about 0.001(10-3) mm of mercury pressure and two electrode were placed on both sides of the tube.
Applying a potential difference between 10,000 volt to 30,000 volt with the help of a high voltage source between the two electrodes. It was found that a glow of green light is produced on the wall in front of the cathode in the glass tube.
This leads to the conclusion that some rays from the cathode fall on the front wall. These rays were named cathode rays by J. J. Thomson. The glass tube in which the power is immersed is called the discharge tube.

Properties of Cathode Rays
William crookes(1879), Hittorf(1889), Perin(1895), J. J. Thomson(1897) and many other scientists studied the properties of cathode rays.
The following are the main properties of cathode rays.
The cathode rays run in straight lines: – The cathode rays form the shadow of an opaque object in its path on the front wall. If an object made of solid metal is placed in the path of cathode rays, its shadow falls on the front wall. This proves that these rays run in straight lines.

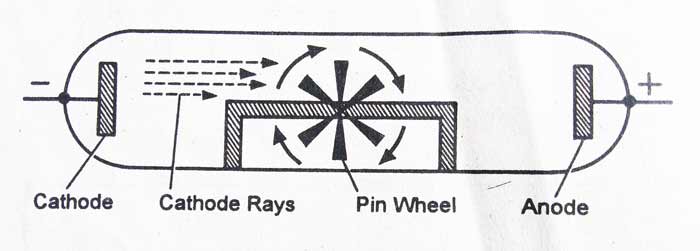
The cathode rays are made up of microscopic particles: – The cathode rays rotates a small and light wheel placed in its path. This proves that these rays are made up of microscopic particles and contain kinetic energy.
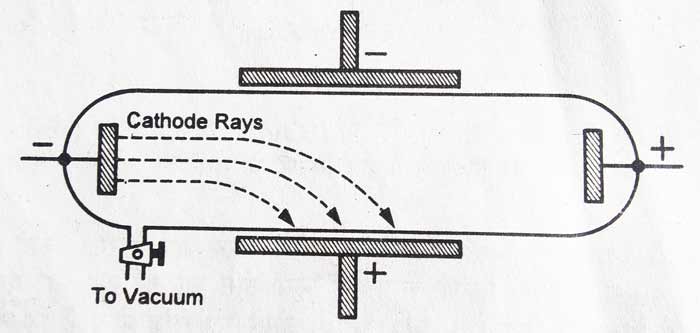
The cathode rays get deflected in the electromagnetic and magnetic fields: – The cathode rays turn towards the positively charged plate in the electric field. In the magnetic field it deflects towards the South Pole. In these experiments it is proved that cathode rays consist of negatively charged particles.
J. J. Thomson found the ratio (e/m) of the negative charge and the amount present on the particles of cathode rays. This ratio does not depend on the substance of the electrodes of the immersion tube or the nature of the gas present in the tube, but is constant in each case.
This leads to the conclusion that the cathode rays obtained from all substances are of the same type and the negatively charged particles present in them are of the same type.
The name of these particles was given ‘electron’ by Stoney and the main credit for its discovery goes to J. J. Thomson. Electrons are present in the atoms of all elements or all substances. They are smaller particles than atoms. Hence electrons are fundamental particles of all Substances or Elements.
That electrons are fundamental particles in all matter has been proven not only by the experiments of electrical immersion in gases but also by studying any other type of phenomena. These are main among them.
Thermoionic Emission: When a substance is heated to high temperature and low pressure, electrons start coming out. This phenomenon is called thermoionic emission.
Photoelectric effect: Even when x-rays, γ-rays or ultraviolet rays collide with metals, electrons start coming out of those metals. This phenomenon is called the photoelectric effect.
Radioactivity: Some rays are automatically emitted from elements like radium, uranium, thorium, etc. These rays are called radioactive rays. And there are three types (α rays, β rays, γ rays). This phenomenon is called radioactivity, out of which β rays consist of electrons.
Ionisation: Legitimate connective compounds dissociate into ions upon contact with water.
NaCl ⇌ Na+ + Cl–
The receipt of positive and negatively charged particles is evidence of the presence of electrons.
J. J. Thomson found the ratio of the charge e on the electron to its mass.
Charge of electron/ mass of electron = e/m
= 1.759 x 108 coulomb per gram
Millikan(1911) found the charge (e) of electron by the oil drop method which was found to be 1.603 x 10-19 coulomb. Since less than this negative charge is not found on any other particle, hence the charge of electron is also called unit negative charge. Hence, mass of electron
m = e /e/m = 1.603 x 10-19 / 1.759 x 108 = 9.1095 x 10-28
According to einstein’s theory of relativity, the mass of a particle varies according to its velocity. If the velocity of a particle is much less than the velocity of light, the mass of the particle is approximately equal to its stop mass. The above mass of electron is known for low velocity electron. Hence, this mass of electron is called its rest mass.
The actual mass of atoms of hydrogen and other elements have been determined with the help of atomic mass and Avogadro’s number. The mass of one atom of hydrogen is 1.6738 x 10-24 grams.
Therefore, the mass of electron is 1/1837 of the mass of hydrogen atom. That is, the mass of electron is much less than the mass of hydrogen atom.
Origin of Cathode Rays
A study of the properties of cathode rays suggests that gases are ionized by the flow of electricity at low pressure in the gases. Electrons and positively charged particles are formed as a result of ionization.
Electrons are attracted to the anode. And as the cathode rays move towards the anode with increasing speed. The positively charged particles move towards the cathode. The positively charged particles take electrons from the cathode to form neutral atoms which are ionized again and produce cathode rays.