Ethyl Amine: Preparation, Properties, Uses, and Tests
Preparation: Laboratory Method
Hoffmann bromide Reaction
Ethyl amine is made from Hoffmann bromide Reaction in the laboratory. Ethyl amine is obtained by heating propionamide with a mixture of bromine and caustic potash. The reaction is completed in the following terms.
C2H5CONH2 + Br2 → C2H5CONHBr + HBr
HBr + KOH → KBr + H2O
C2H5CONHBr + KOH → C2H5NCO + KBr + H2O
C2H5NCO + 2KOH → C2H5NH2 + K2CO3
The complete reaction can be represented by the following equation.
C2H5CONH2 + Br2 + 4KOH → C2H5NH2 + 2KBr + K2CO3 + 2H2O
Preparation Method
In a round bottom flask, take a mixture of about 40 grams of propionamide and about 36 ml of bromine. Keep the flask in ice cold water. A drop of 10% caustic potash solution is found in such a quantity in this mixture that the reddish brown colour of bromine is removed.

Now, keep the flask on water-heat and arrange and slowly pour about 50 ml 50% caustic potash solution from the point funnel. After this, the flask is heated by removing it from the water heater and placing it on a wire mesh, and the vapours of ethyl amine are absorbed in hydrochloric acid kept in the receiver.
Basic Concepts of Amines
- 1. Introduction
- 2. Classification
- 3. Nomenclature
- 4. Structure
Pure ethyl amine is obtained after distillation by adding caustic potash to the solution obtained in the receptor.
Huffman Alkalization method
In this method hydrogen atoms of ammonia are replaced by alkyl group. By this method, ethyl iodide is heated with a concentrated ammonia or alcohol solution of ammonia in a closed tube to obtain ethyl amine. The reaction of ethyl iodide and ammonia results in ethyl amine.
C2H5I + NH3 → C2H5NH2 + HI
In fact, a mixture of primary, secondary, and tertiary amines and quaternary ammonium salts is obtained in this method.

Hence this method is not suitable for making ethyl amine in the laboratory. In industries, fractional distillation by adding alkali to this mixture. The primary, secondary, tertiary amines isolates are obtained as a result of efficient distillation.
Ethyl Alcohol
Ethyl amine is obtained when a mixture of vapors and ammonia of ethyl alcohol flows over 360°C alumina.
C2H5OH + NH3 → C2H5NH2 + H2O
In this method also a mixture of primary secondary and tertiary amines is obtained, but when used ammonia intensively, the main product is either primary amine or ethyl amine.
By reduction of acetone, methyl cyanide, nitro Ethan and acetaldehyde oxime:
reaction of these compounds with H2 – Ni, Na – C2H5OH, LiAlH4, NaBH4 and some other catabolites gives ethyl amine.
CH3CONH2 + 4H → CH3CH2NH2 + H2O
CH3CN + 4H → CH3CH2NH2
C2H5NO2 + 6H → C2H5NH2 + 2H2
CH3-CH=NOH + 4H → CH3CH2NH2 + H2O
Water decomposition of ethyl isocyanide
Ethyl amine is obtained by water decomposition of ethyl isocyanide or ethyl isocyanate. These reactions are catalyzed by acide and alkali respectively.
C2H5NC + 2H2O → C2H5NH2 + HCOOH
C2H5NCO + 2KOH → C2H5NH2 + K2CO3
Grignard Reagent
ethyl amine is obtained by the action of chloramine on ethyl magnesium chloride, bromide or iodide.
C2H5MgCl + ClNH2 → C2H5NH2 + MgCl2
Schmidt Reaction
Ethyl amine is obtained by reaction of propionic acid and hydrazoic acid in the presence of concentrated sulphuric acid. This reaction is called Schmidt Reaction.
C2H5COOH + N3H → C2H5NH2 + CO2 + N2
Physical Properties
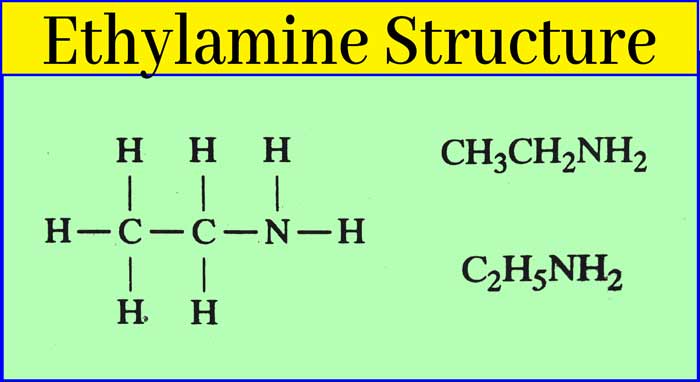
Ethyl amine is a colorless liquid. Its boiling point is 19°C. Hence, it is colorless gas in summer season. It smells like ammonia. It is soluble in water. And its aqueous solution is alkaline. IUPAC name of ethylamine is Ethanamine.
Chemical Properties
Alkaline properties: It dissolves in water to form ethyl ammonium which is an alkali.
C2H5NH2 + H2O → C2H5NH3+OH–
It reacts with acids like ammonia to form salts.
C2H5NH2 + HCl → C2H5NH3+Cl–
C2H5NH2 + H2SO4 → C2H5NH3+HSO4–
It reacts with chloroplatinic acid (H2PtCl6) to form platinichloride salts.
2C2H5NH2 + H2PtCl6 → (C2H5NH3+)2(PtCl6)2-
On burning of platinichloride salts, only the platinum remains and the remaining elements separate as gas.
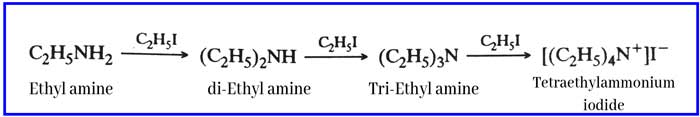
Reaction with alkyl halides: It reacts with alkyl halides to form a mixture of secondary amine, tertiary amine and quaternary ammonium salts.
Example: with ethyl iodide
Acetylation: It reacts with acetyl chloride or acetic anhydride to form N-substituted acetamide. In this reaction a hydrogen atom of the – NH2 group is replaced by an acetyl (-COCH3) group. This reaction is called acetylation.
C2H5NH2 + CH3COCl → CH3CONHC2H5 + HCl
C2H5NH2 + (CH3CO)2O → CH3CONHC2H5 + CH3COOH
Using a greater amount of reagent – both hydrogen atoms of NH2 group are replaced by two acetyl groups.
Reaction with nitrous acid: It reacts with nitrous acid to make ethanol and nitrogen is gas free.
C2H5NH2 + HNO2 → C2H5OH + H2O + N2
The -NH2 group present in any compound is converted into -OH group with the help of this reaction. Nitrous acid is a temporary acid. To make this reaction, a mixture of sodium nitrite and hydrochloric acid is used. The reaction of sodium nitrite and hydrochloric acid results in the formation of nitrous acid.
The secondary amine reacts with nitrous acid to form N-nitroso-amine, which gives yellow oily layer.
The teritery amine reacts with nitrus acid to form soluble salts and there is no visible change.
- Ethyl Amine: Preparation, Properties, Uses, and Tests
- Amines: Nomenclature, Isomerism, Basic Characters
- How is Ethyl Acetate made? | Properties | Uses
- Is Acetamide base or acid? | Preparation | Properties | Uses
- Acetic Anhydride: What is acetic anhydride used for?| Preparation | Properties
- Acetyl chloride: How do you make Acetyl Chloride? | Properties | Uses
- Carboxylic Acid: What is the common name of a carboxylic acid?
- Oxalic Acid: What is the formula of oxalic acid? | Properties | Uses
Reaction of amines to nitrous acid is used for their testing and differential detection.
Carbylamine reaction: On heating primary amines with an alcohol solution of chloroform and KOH, a highly deodorant additive called iso cyanide or carbylamine is formed. This reaction is called carbylamine reaction and this reaction is used to test primary amine. The following is the equation of the carbylamine reaction of ethyl amine.
C2H5NH2 + CHCl3 + 3KOH → C2H5NC + 3KCl + 3H2O
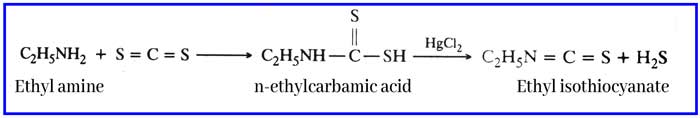
Reaction with carbon di sulfide: N – ethyl di thao carbamic acid is formed by the reaction of ethyl amine and carbon disulfide. On reaction with its mercuric chloride, ethyl iso-thao-cyanate is formed which has a smell similar to mustard oil. This reaction is called haffman mustard oil reaction. And this reaction is used to test ethyl amine.
Grignard reagent reaction: Reaction of ethyl amine to grignard reagents results in alkane.
CH3MgI + C2H5NH2 → CH4 + C2H5NHMgI
C2H5MgBr + C2H5NH2 → C2H6 + C2H5NHMgBr
Reaction with benzen sulfonil chloride: The reaction of primary amines with benzene sufonil chloride produces N – alkyl benzene sulfonamide, which is dissolved in dilute alkaline solution. Following is the equation of the reaction of ehtyl amine and benzene sulfonil chloride.
C6H5-SO2-Cl + C2H5NH2 → C6H5-SO2-NH-C2H5
Reaction of secondary amine with benzene sulfonil chloride produces N, N – di alkyl benzene sulfonamide, which are insoluble in alkaline solutions.
There is no reaction of benzene sulfonil chloride of teritery amines.
The reaction of amines with benzene sulfonil chloride is used to separate them from a mixture of primary, secondary and teritery amines. This method of separating them from the mixture of primary, secondary and teritery amines is called hinsberg method.
Ethylamine Tests
Ethyl amine and other alifatic primary amines give the following tests.
Nitrous acid test: Reaction occurs rapidly when sodium tritrite and hydrochloric acid are added to the amines compound and nitrogen gas is produced. nitrogen gas exits rapidly with bubbling.
Carbylamine test: On heating the amines additive with chloroform and alcoholic caustic potash solution, a strong deodorant is obtained.
Haffman mustard oil test: The amines additive receives a mustard oil-like odor when heated with carbon di sulfide in the presence of mercuric chloride.
Ethylamine Uses
It is used as a production raw material of herbicides like Cyanazine, Atrazine and Simazine. Ethylamine is used as a catalyst in end use industries such as plastics for production of polyurethane foam and in dyes for producing ethylcyanopyrolidone disperse and rhodamine dyes.