How to test for Formaldehyde? Preparation, Properties and uses
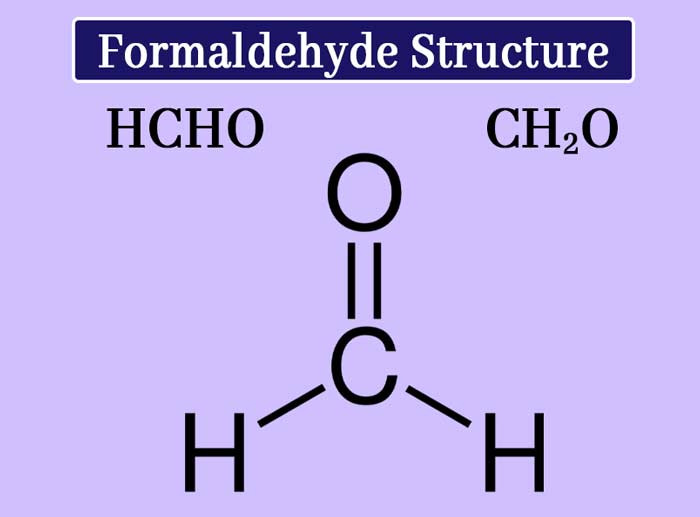
This compound is the first member of the aldehyde. Formaldehyde s molecule formula CH2O and structure formula are as follows.
 Formaldehyde Preparation
Formaldehyde Preparation
Laboratory Method
In the laboratory it is obtained from the oxidation of methyl alcohol. In this method, the mixture of methyl alcohol vapor and air is flowed on 200 – 300°C heated platinised asbestos.
You can also use copper lattice heated to 300°C in place of platinised asbestos. Formaldehyde is obtained as a result of the following reaction.
2CH3OH + O2 – Pt.–Asbestos → 2HCHO + 2H2O
Method: Take methyl alcohol in a round-bottomed flask and put a two-hole cork in it. Air enters the flask from one hole and the other hole is connected to the right tube in which the platinised asbestos is kept.
The other end of the burning tube is connected to the receptacle filled with water. The receiver is fitted with a suction pump.
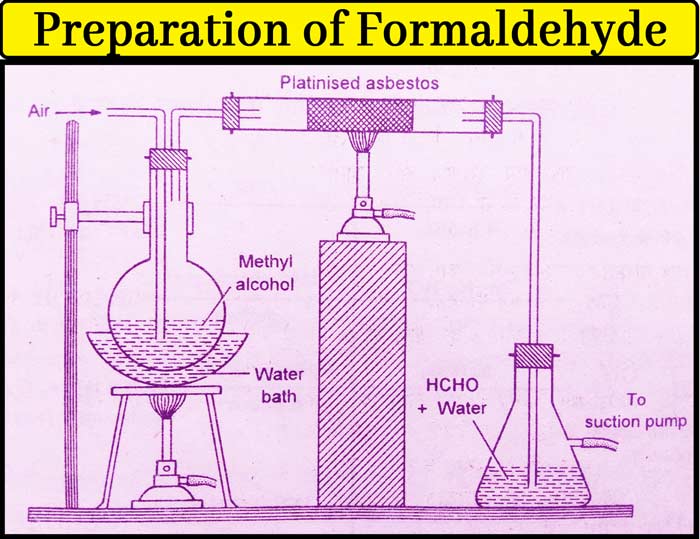
Heat the flask to 40°C on a water heater. The platinised asbestos are heated to 200°C. Vapor of methyl alcohol is also drawn when air is drawn from the suction pump into the flask.
In the presence of a catalyst present in the burning tube, it is oxidized by oxygen of air to form formaldehyde which dissolves in the water kept in the receiver. In addition to formaldehyde and water, small amounts of methyl alcohol are also in the solution obtained from the receptor.
The methyl alcohol is separated by distillation. In the end, the solution found contains about 30% formaldehyde and the remaining water. This is called formalin. Formaldehyde is often used in this form.
Industrial Method
The following are the major methods of industrial manufacture of formaldehyde –
From the hydrogenation of methyl alcohol:
CH3OH – Ag/300° → HCHO + H2
From methyl alcohol catalytic oxidation:
2CH3OH + O2 – Ag/450° → HCHO + 2H2O
By air oxidation of methane:
CH4 + O2 → HCHO + H2O
Physical Properties
- Formaldehyde is a colorless and pungent odor gas.
- On cooling, it is converted into a liquid with a boiling point of -21°C.
- It is soluble in water, ether, and alcohol.
Chemical Properties
Please check following Post for Chemical Properties of formaldehyde
- How will you distinguish between aldehyde and ketone?
- Aldehydes and Ketones: Preparation, Properties, Nomenclature
Formaldehyde is a gas at normal temperature. About 30% of its aqueous solutions are called formalin. Formaldehyde is often used only as formalin. Its major uses are as follows.
- For preservation of biological and anatomical specimens
- as antiseptic
- In the manufacture of artificial colors
- In place of tanin in leather industry
- Plastic is made from formaldehyde such as bakelite galalith, formaldehyde urea.
- Urotropine derived from the action of formaldehyde and ammonia is used in urinary diseases.
- Sucking pills called formalin tablets are made from formaldehyde and lactose.
Formaldehyde gives all the normal tests of the aldehyde group. It gives pink color with schiff reagent, silver mirror with tollan reagent and red precipitate with fehling solution.
Specific testing of formaldehyde is as follows.
A drop of 0.5% aqueous resorcinol solution is found in 1 ml of dilute aqueous solution of formaldehyde. This mixture is slowly found in the surface of 5 ml of concentrated H2SO4. Carefully stir the test tube in such a way that both the surfaces are not lost. A ring of red violet is formed at the meeting of both surfaces.
Add 1 ml of 5% potassium pherocyanide solution to 3 ml of 1% phenyl hydrezine hydrochloride solution. In this solution, some droplets of formaldehyde solution are mixed with few drops of concentrated HCl. The color of the solution turns red. This test is called schryvers test.
A dilute solution of formaldehyde consists of a few drops of 1% phenyl hydrazine hydrochloride solution and a few drops of sodium nitroprusside solution. Dark blue color is obtained when this solution is alkalized with caustic soda. It changes color, first being greenish brown and finally red. This test is called rimmis test.
