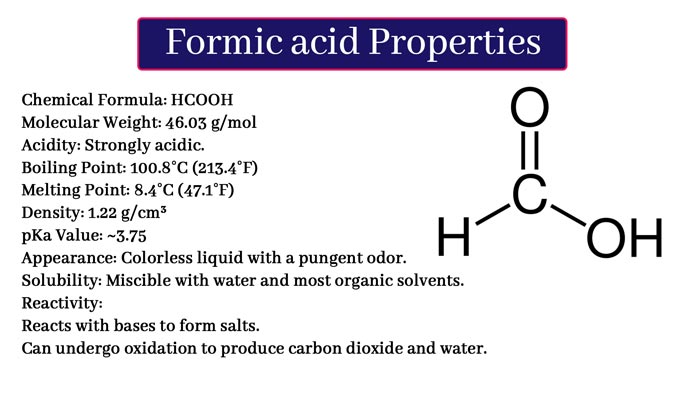
Formic Acid: Preparation, Properties, Uses and Tests
Formic acid is the first member of the Carboxylic Acid Group. It is found in red ants, honey flies, wasps and grass scorpions.
The itching or burning that occurs in the body due to the bite or sting of these organisms is due to the entry of formic acid into the body.
It is also found in small amounts in urine, sweat and meat extract. Its molecular formula is CH2O2. Following is its structure formula.

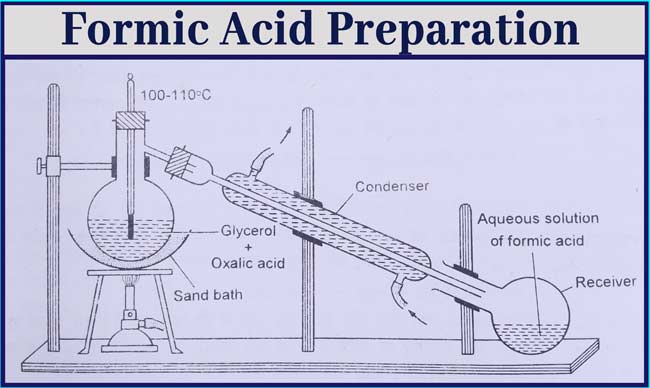
Formic Acid Preparation
Laboratory Method
From glycerol and oxalic acid: Formic acid is obtained by heating a mixture of glycerol or oxalic acid to 100° – 110°C temperature.
Take about 40 grams of crystal Oxalic Acid and 50 ml Anhydrous glycerol in a distillation flask and heat it slowly at 100°- 110°C with the help of a sand boiler.
Aqueous solution of formic acid is distilled and collected in the receptor. After the reaction is over, the glycerol remains in the distillation flask.
Formic acid can be obtained again by heating and adding oxalic acid again in the distillation flask. The reaction is completed in the following three terms.
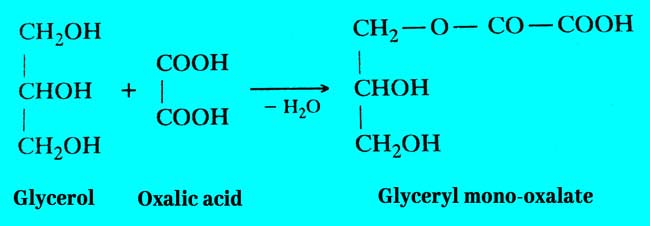
Firstly glycerol and oxalic acid combine to form glycerol mono oxalate.
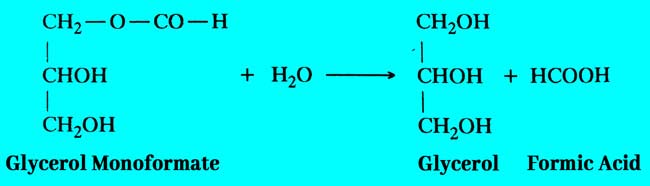
By heating glycerol mono oxalate to 100°C temperature, one molecule of carbon dioxide is separated from it and it converts to additive glycerol monoformate.

Glycerol Monoformate reacts with water derived from the first term of this reaction and crystal of oxalic acid to form Formic acid and Glycerol.
In this reaction only a small amount of glycerol gives bad amounts of oxalic acid to formic acid.
Method of obtaining anhydrous formic acid from an aqueous solution of formic acid: Some amount of water is present in formic acid obtained by laboratory method.
Since formic acid has a boiling point of 100, it cannot be dehydrated by the efficient distillation method.
Therefore, to make it anhydrous, the water containing acid is heated by neutralizing lead carbonate and the hot solution is sieved with the help of hot water funnel.
On cooling this filtered fluid, lead becomes crystal of Lead Formate, which are separated and dried.
2HCOOH + PbCO3 → (HCOO)2Pb + H2O + CO2
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
Dry crystals are placed in the inner tube of a condenser. The lower end of the condenser is connected to the receiver and the upper end is connected to the Kip device. Now the crystals are heated by steam and flow hydrogen Sulfide from the capacitor to the Kip apparatus. On doing this, formic acid and lead sulfide are formed.
(HCOO)2Pb + H2S → 2HCOOH + PbS
Formic acid is obtained in the receptor. And the solid lead sulfide remains in the tube itself. Thus small amounts of hydrogen sulfide in formic acid are impaired. This can be overcome by distillation with small amounts of lead formic. The formic acid obtained in this way is pure and anhydrous.
Industrial Method
From carbon mono oxide: Sodium format is obtained by heating a mixture of carbon mono oxide and sodium dioxide at 150°C heat and about 8 atmospheric pressure.
CO + NaOH → HCOONa
Formic acid is obtained by adding appropriate amounts of sulfuric acid to the sodium format.
HCOONa + H2SO4 → HCOOH + NaHSO4
Methyl Alcohol: Formic acid is obtained by oxidation of methyl alcohol.
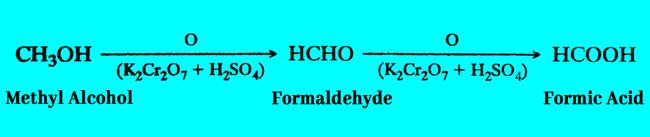
Formaldehyde: formic acid can also be obtained by oxidation of Formaldehyde according to the second term of the above equation.
Hydrogen Cyanide: Formic acid is obtained from the water decomposition of hydrogen cyanide.
HCN + 2H2O → HCOOH + NH3
Methyl Format: formic acid can be obtained by water decomposition of methyl format.
HCOOCH3 + H2O → HCOOH + C2H5OH
Physical Properties of Formic Acid
It is a colorless, pungent, odor and hygroscopic fluid. Its boiling point is 100.5°C. It is a fusion of water, ether and alcohol in all proportions. It forms blisters on the skin as it is an intense corrosive.
Chemical Properties of Formic Acid
Formic acid is the first and simplest member of the carboxalic acid group. In it, a – COOH group is attached to an H atom.
Other members of this class include the -COOH group associated with the alkyl group or another group. It exhibits reducing properties due to the presence of formic group.
It also decomposes easily compared to other carboxalic acid. For these reasons it is more functional than other members of this group.
The following are the major chemical reactions of formic acid.
Reducing Properties: Like aldehydes it is a good reducing agent due to the presence of formic group in it. This ammonium silver nitrate (Tollen’s reagent) reduces the acidic solution of fehling solution, mercuric chloride and potassium permanganate.
HCOOH + Ag2O → CO2 + H2O + 2Ag↓
HCOOH + 2CuO → Cu2O↓ + CO2 + H2O
HCOOH + 2HgCl2 → Hg2Cl2↓ + 2HCl + CO2
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5O
HCOOH + O → CO2 + H2O
Other members of the alkanoic acid series do not display this property.
Acidic Properties: It is the most acidic compared to its category of acids. It is about 10 times stronger than acetic acid. It exhibits all the common properties of acids. It forms salts with alkali. And esters are formed with alcohols.
HCOOH + NaOH → HCOONa + H2O
2HCOOH + Na2CO3 → 2HCOONa + CO2 + H2O
HCOOH + NaHCO3 → HCOONa + CO2 + H2O
2HCOOH + Ca(OH)2 → (HCOO)2Ca + 2H2O
HCOOH + C2H5OH → HCOOC2H5 + H2O
It also forms salts by reacting with metals like sodium, zinc etc. And hydrogen gas comes out.
Example:
2HCOOH + 2Na → 2HCOONa + H2
Decomposition – Heating at 160°C temperature under the influence of pressure decomposes it into CO2 and H2.
HCOOH → CO2 + H2
Dehydration: Carbon mono oxide and water are formed when heated with concentrated sulphuric acid.
HCOOH → CO + H2O
Reaction of phosphorus pentachloride: By reacting with phosphorus pentachloride, formic acid formyl chloride (HCOCl) is formed which decomposes into carbon mono oxide and hydrochloric acid due to temp.
HCOOH + PCl5 → HCOCl + POCl3 + HCl
HCOCl → CO + HCl
Reaction of Thionyl chloride: This acid acts like PCl5 with thionyl chloride.
HCOOH + SOCl2 → HCOCl + SO2 + HCl
HCOCl → HCl + CO
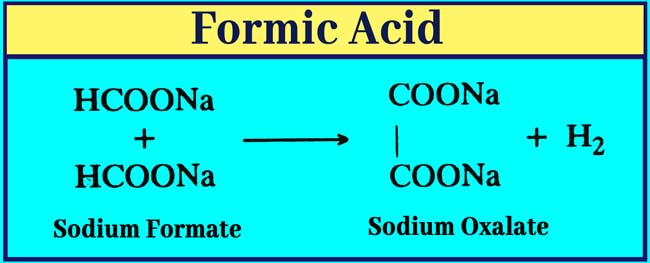
Effect of heat on salts: Sodium oxilate and hydogen are formed by heating sodium or potessium format to 360°C. Salts of other carboxilic acid are stable at this temperature.
Hydrogen gas is released when sodium or potassium format is heated with soda lime. While other alkenoic acids make alken.
HCOONa + NaOH → H2 + Na2CO3
Formamide is formed upon heating the ammonium format.
HCOONH4 → HCONH2 + H2O
Formaldehyde is formed by dry distillation of calcium format.
(HCOO)2Ca → HCHO + CaCO3
Dry distillation of calcium format with calcium acetate produces aceteldihyde.
(CH3COO)2Ca + (HCOO)2Ca → 2CH3CHO + 2CaCO3
Formic Acid Uses
In dyeing woolen and cotton clothes.
In coagulation of latex.
Leather tanning
Create a nickel format. It is used as a catalyst in the hydrogenation of oils.
In the manufacture of gout medicines.
Formic Acid Tests
All carboxylic acids give the following general tests.
It becomes red when a blue litmus sheet is added to an aqueous solution of carboxylic acid.
A saturated solution of NaHCO3 in carboxylic acid yields effervescences due to the formation of carbon dioxide.
In addition to the above, the following are typical tests of formic acid.
Silver mirror is obtained by heating ammoniuam silver nitrate in formic acid.
Red precipitate is formed due to cuprus oxide being formed when heated with a fehling solution in formic acid.
White
precipitate is formed due to the formation of mercurus chloride when
formic acid is heated with mercuric chloride solution.
It is neutralized by ammonium hydroxide and a red color is obtained by adding a few drops of feric chloride solution.
On heating this acid with concentrated sulfuric acid, carbon monoxide gas is released which burns with flame.