Fructose: How Fructose Converted into Glucose? | Structure and Formula
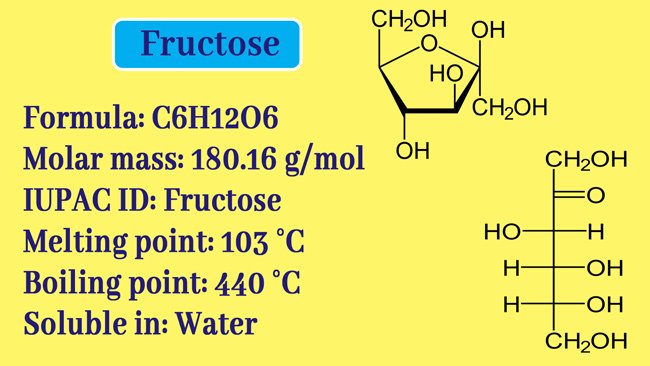
Like glucose, fructose is also a mono saccharide. And fructose formula is C6H12O6. But glucose contains aldehyde group while fructose contains ketone group. It is found in fruits and honey in the Free State in nature and in the form of sucrose in the combined state with glucose.
It is also called fruit sugar because it is found in fruits. Natural fructose is laevorotatory, so it is also called l-fructose, d-(-) fructose or levulose.
Preparation of Fructose
From Sucrose
fructose is obtained from water decomposition by dilute mineral acids (HCl or H2SO4) of sucrose.
C12H22O11 + H2O → C6H12O6 + C6H12O6
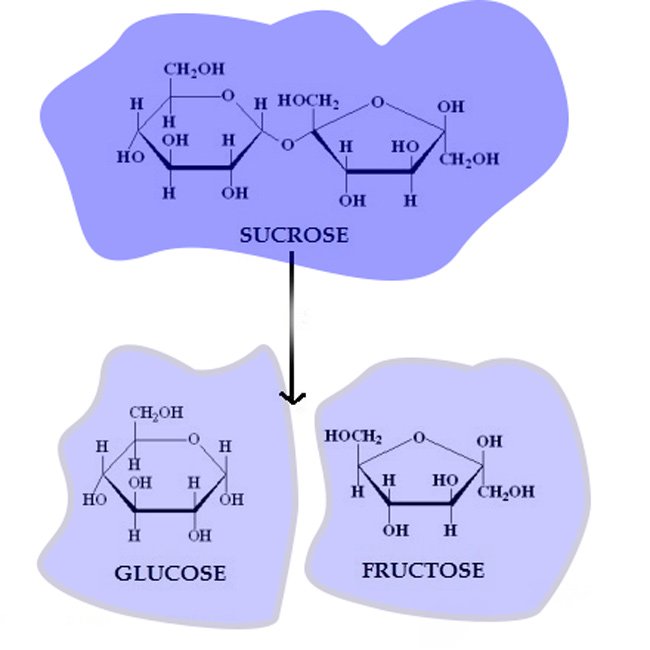
This reaction is carried out in alcohol solution, glucose and fructose are obtained separately after the crystallization of the solution obtained.
From Inulin
Inulin is a poly saccharide found in many plants in nature. Fructose is obtained by water decomposition by its dilute H2SO4.
(C6H10O5)n + nH2O → nC6H12O6
The obtained solution is replicated with the barium hydroxide solution. White precipitate of barium sulfate is obtained, which is filtered and separated. Fructose crystals are obtained upon concentration of the remaining solution.
Physical Properties of Fructose
Fructose is a white cristal solid. Its melting point is 103°-105° C.
Of all the sugars, fructose is the most commonly consumed sugar.
Its sweetness is about 2 times the same amount of sweetness as sucrose.
It is more soluble in water and is soluble in ether. Natural fructose is laevorotatory. fructose also exhibits variable mutarotation properties similar to glucose.
The specific mutarotation of its fresh solution is -21° or -133° which after some time becomes -92°. Fructose formula different from glucose formula.
Fructose Chemical Properties
The molecule formula of Chemical: fructose formula is C6H12O6. Its structure is represented by formula (I), while its actual composition is formula (II).
It is clear that five hydroxy and one ketone group are present in one molecule of fructose and in fructose the ketone group is not present in free form but in hemiacetal form.
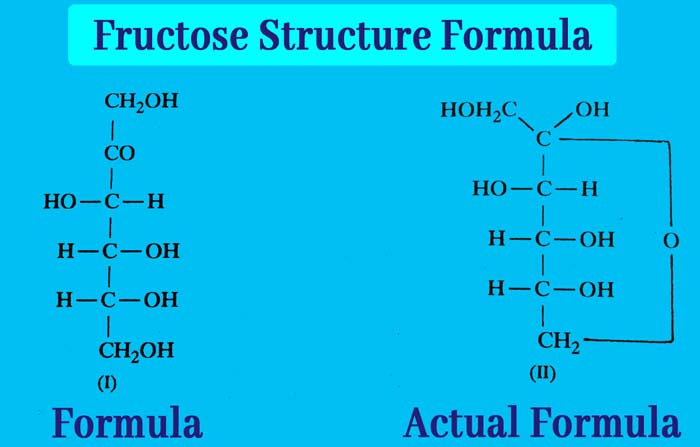
Comparing the structures of glucose and fructose, it is clear that the group of four carbon atoms and their configuration is similar. There is a variation in the group associated only with carbon atoms 1 and 2.

The major chemical reactions of fructose are given below.
Action of sodium hydroxide: Reaction of fructose with dilute sodium hydroxide results in polarization mixture of D – (+) – glucose, D-(+)- mannose, D-(-)-Fructose. This reaction is called Lobry de bruyn van ekestein rearrangement.
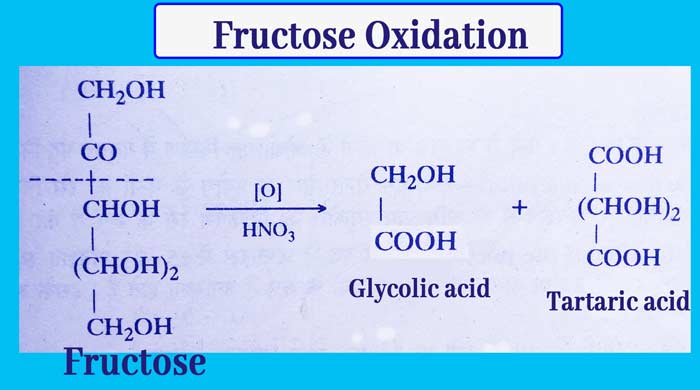
Fructose Oxidation: Bromine water allows glucose to oxidation to gluconic acid but there is no reaction with its fructose.
Fructose + Bromine water → No Reaction
This reaction is used to distinguish glucose and fructose.
Due to the presence of aldehyde group in glucose, it exhibits reducing properties and reacts easily with weak oxidants such as tollan reagent and fehling solution.
Due to the presence of ketone group in fructose, it does not exhibit reducing properties and does not react with weak oxidants, however fructose gives positive testing with tollan reagent and fehling solution.
The reason for this is that fructose is reabsorbed into glucose in the alkali subsoil present in both of these reagents, and silver mirror and brown precipitates are obtained, respectively, as a result of the reaction of glucose to tollan reagent and fehling solution.
Fructose Reduction: Reduction of fructose by H2–Ni, Na-C2H5OH, LiAIH4 or NaBH4 results in two timely hexahydric alcohol.

Phenylhydrazine: Like glucose, fructose also reacts with excess of fenil hydrezine to form osazone. The time to form osazone by the reaction of fructose and Phenylhydrazine is 5 minutes and the melting point of osazone is 205°C.
In the reaction of glucose and Phenylhydrazine, CH2OH group is oxidized to ketone group. In the reaction of fructose and Phenylhydrazine, CH2OH group is oxidized to aldehyde group.
Fermentation: Like glucose, fermentation takes place in an aqueous solution of fructose in the presence of an enzyme called Zymase and Ethyl alcohol is obtained.
C6H12O6 – zymase → 2C2H5OH + 2CO2
Uses of Fructose
Making sweet pills and candies
In creating syrup of medicine
As an alternative to table sugar