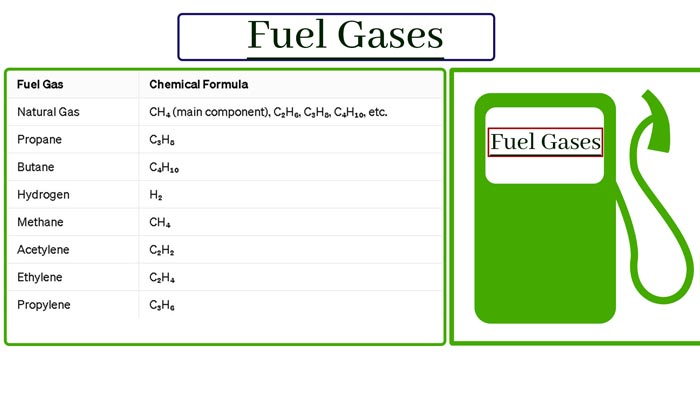
Fuel Gases: What type of fuel is gas?
Fuel Gas : The substances in which such ingredients are present which produce heat by acting with oxygen are called fuels.
In order to obtain heat from the fuel, initially it is given a small amount of heat in the presence of oxygen from some other source, by doing this, a microscopic part of the fuel reacts with oxygen and in this action heat is produced, which first Exceeded heat is given.
In this way, one part of the heat generated becomes radiate and the remaining part is helpful in reacting oxygen to another part of the fuel. Thus heat is generated and the fuel is slowly destroyed, this action is called burning of fuel.
Fuel Gas System
Fuel + Oxygen → Products + Heat
The amount of heat that a calorie produces from burning one gram of a fuel is called the calorific value of that fuel. The higher the calorific value of a fuel, the better the fuel is considered to be. The calorific value of carbon is 7830 calories per gram.
There are three types of fuel solid, fluid, gas, wood and coal are the main fuel. Among the liquid fuels, kerosene petrol and diesel are the main ones. Oil gas petrol gas producer gas water gas and call gas are major in gas fuel. Gaseous fuel is more useful than solid and liquid fuels.
Why we use Fuel Gases
- They burn smoothly and leave no residue
- Smoke emits or does not emit at all from their burning.
- They have high Calorific value
Oil Gas
Oil gas contains simple hydrocarbon gases such as methane, ethylene, acetylene, and propylene. Oil gas is made from kerosene.
Kerosene is a mixture of high-weight hydrocarbons. Hydrocarbons with high concentrations are usually liquid and hydrocarbon gases with low concentrations.
On heating the hydrocarbons with high loads in the absence of air, they are separated into hydrocarbons with less charge. This action is called cracking. This process is used to obtain oil gas from kerosene.
To make oil gas from kerosene, pour kerosene drop by drop into a heated and closed retort. Kerosene cracking occurs on doing this. And collect oil gas. The hydraulic Siphon acts as a filter and prevents unnecessary side products from entering the gas tank.

Uses of Oil Gas
The major use of oil gas is in the laboratory as fuel. In the laboratory, heat is obtained by mixing it with air and burning it in burners.
There is three part of Bunsen Burner.
- Base
- Air vents
- The fireplace
The flame that is received when the air hole is opened is called non-luminous flame.
It has a blue section around it and a lightless part. Gaseous hydrocarbon and air are present in the blue part. Gaseous hydrocarbono is combusted in the lightless part.
Example:
2C2H6 + 7O2 → 4CO2 + 6H2O + Heat
Unused oxygen remains in the lightless part. Therefore, this flame is also called oxidising flame. The temperature between the lightless part of the flame is maximum. Therefore, the temperature above the blue part is maximum. Lightless flame is used in most of the works.
The flame received after closing of the air holes is called the luminous flame. It has a blue section whose size is relatively small. There is a yellow part around the blue part.
Unburned carbon is present in the yellow part. Therefore, this flame is also called a reducing flame. On heating a vessel in this flame, the carbon in its bottom freezes and the bottom becomes black. Lucent Flame Heating Prkashhin lower flame heating.
Petrol Gas
Petrol is also a mixture of hydrocarbons with high concentrations like kerosene but the presence of petrol hydrocarbons is less than the hydrocarbons present in kerosene. Hence, petrol is a more volatile liquid than kerosene.
Most of the petrol is evaporated by applying a strong gust of air in the petrol. The mixture of petrol vapours is called petrol gas. The mixture of gases obtained when petrol is broken down like kerosene is also called petrol gas.
Like oil gas, petrol gas is also used as a fuel in the laboratory. In the laboratory, heat is obtained by burning the mixture with its air in burners.
Producer Gas
Producer gas mainly consists of carbon mono oxide and nitrogen gases. The ratio of carbon mono oxide and nitrogen in it is about 1: 2 considering the volume.
To make this, they fill the coke of the furnace and burn it and keep the air flowing from the bottom of the furnace. On doing this, the temperature of coke reaches about 1100°C.
C + O2 → CO2 + 94 kcal
CO2 + C → 2CO – 40 kcal
2C + O2 → 2CO + 54 kcal
While collecting the producer gas, the temperature of coke should not be less than 1100°C, otherwise the amount of CO2 in it will be high and its calorimeter will be reduced.
Producer gas is used as a gaseous fuel. It is used in cement and Martian open floor furnaces.
Water Gas
Water gas is mainly a mixture of carbon mono-oxide and hydrogen gases. It contains about 50% carbon mono-oxide, about 40% of hydrogen and about 10% of other gases(O2, N2, CH4, etc).
To make it, fill the coke in a furnace and burn it and keep the air flowing down the furnace. This stops the flow of air and causes water vapor to flow. Water gas is obtained by this action.
H2O + C → H2 + CO – 29Kcal
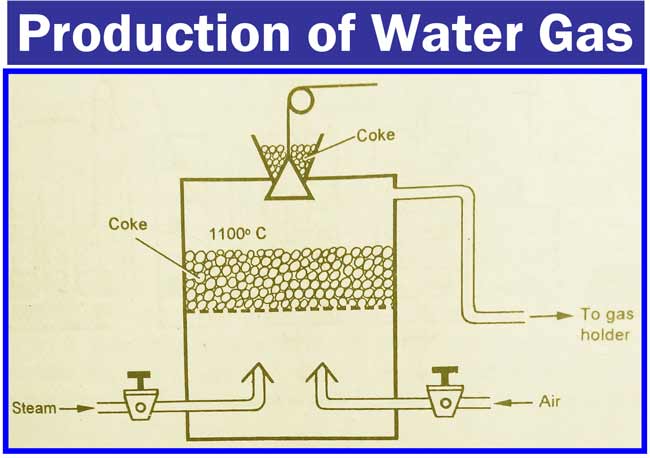
Due to the endothermic nature of the above action the temperature of the furnace starts to decrease. The action of C and H2O at low temperature increases the speed of CO2 production and reduces CO production.
Therefore, after a short period of time, the water vapor in the furnace stops flowing and the air flows until the coal is white-heated. The temperature of the furnace reaches 1100, after which the water vapor can flow again and water Gas is obtained.
It is clear that the heat of coke must be high otherwise the water gas obtained will have a higher ratio of carbon di-oxide which will reduce its caloric value.
Uses of Water Gas
4CO + 4H2 + Ni → Ni(CO)4 + 4H2↑
Water gas is a major gaseous fuel. Its calorific value is very high. It is also used in the manufacture of hydrogen, carbon mono-oxide and methane alcohols and as a reducing agent.
In order to make carbon mono-oxide from water gas, it first flows on about 50% nickel metal.
By heating obtained nickel carbonyl to 180°C, the nickel is separated and carbon mono-oxide gas is obtained.
Ni(CO)4 → Ni + 4CO↑
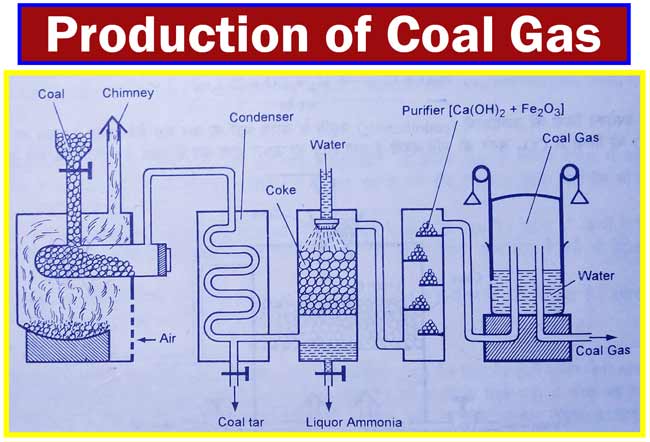
Coal Gas
Coal gas is formed by destructive distillation of coal. It mainly consists of hydrogen, methane and carbon mono-oxide gas. Its composition depends on the coal sample and the temperature of the distillate. Its quantitative organization is as follows –
- Hydrogen(H2): 45 – 55%
- Methane(CH4): 25 – 35%
- Carbon Mono-Oxide (CO): 4 – 11%
- Other Hydrocarbon: 2 – 5%
- Other gases (N2, CO2, O2): 2 – 15%
On burning coal gas, hydrogen and carbon mono-oxides mainly generate heat. Methane produces heat and light and other hydrocarbons mainly produce light.
For the manufacture of coal gas, the coal powder is heated to about 1000°C in the absence of air in a fire-retarded retort. The received gases are cooled by a condenser.
Here coal tar is obtained as a side product. The gases from the condenser are sent downstream to a washing tower. This column is filled with coke and water is given from above.
Ca(OH)2 + CO2 → CaCO3 + H2O
Ca(OH)2 + 2H2S → Ca(HS)2 + 2H2O
Ca(HS)2 + CS2 → CaCS3 + H2S
Fe2O3 + 3H2S → Fe2S3 + 3H2O
Fe2O3 + 6HCN → 2Fe(CN)3 + 3H2O
Ammonium liquid is obtained from this column as a lateral product. The gases obtained from this column are sent to a compact chamber where the extinguished and moist Fe2O3 is kept. Here the impurities of CO2, HCN, H2S and CS2 are removed.
The gaseous mixture obtained from the purification chamber is collected over the water in the inverted reverse tanks.
The main use of this gas is to obtain heat as a gaseous fuel. This gas is also used to generate light. This gas is also used as a reducing agent and to produce an inert atmosphere.
Liquefied Petroleum Gas
Liquefied petroleum gas is abbreviated to L.P.G. It is said. Where petroleum is found there exists a gas atmosphere with petroleum floating within the earth. Which is called natural gas.
When petroleum wells are dug during petroleum mining. So natural gas is first available. With the help of suction pump, natural gas is separated. Natural gas contains C1 – C6 alkanes.
It contains about 80-90% methane gases and the remaining C2 – C6 alkanes. Natural gas is liquefied under the influence of pressure, filling this fluid in the cylinder. And it is used in the name of liquefied natural gas as fuel in homes.
When natural gas is liquefied under pressure, this action can also be performed in two terms. The fluid obtained in the first term consists mainly of propane and butane gases and a very small amount of C5 – C6 alkanes.
After this, the remaining gas is also liquefied by increasing the pressure. And the fluid obtained in the second term mainly consists of methane and ethane gases.
The fluid obtained in the first term is liquefied petroleum gas or L.P.G. It is said. Fill it in the cylinder. Its major use is as a fuel in homes. It has very high calorific value. Its calorific value is around 100 KJune per litre. The fluid obtained in the second term is mainly used in the manufacture of petrochemicals.
Liquefied petroleum gas mainly consists of propane and butane gases and is derived mainly from natural gas. Other processes in liquified petroleum industry, such as propane and butane gases obtained from carcking, are also obtained by liquefying liquids under the influence of pressure. It is said. And it is used as fuel in homes.