Glucose Chemical Reaction || Glucose Chemical Formula and Properties
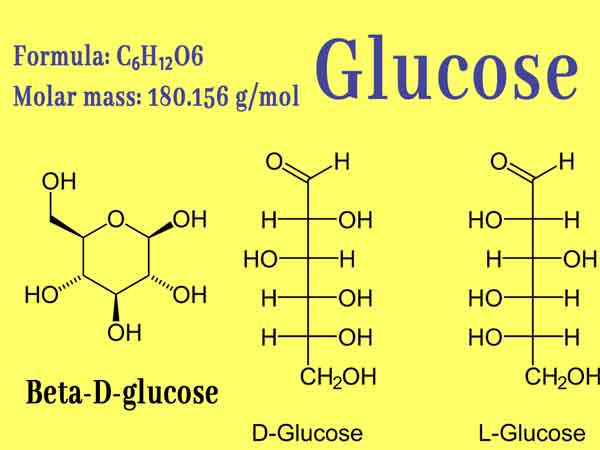
Glucose, organic compound, molecular formula C6H12O6. It is the most widely distributed and most important monosaccharide in nature, and it is a polyhydroxy aldehyde. Pure glucose is a colorless crystal with sweet taste but not as sweet as sucrose, easily soluble in water, slightly soluble in ethanol, and insoluble in ether. The natural glucose aqueous solution rotates to the right, so it belongs to “dextrose “. Glucose has an important status in the biological field, and it is the energy source and metabolic intermediate product of living cells, that is, the main energy-supplying substance of organisms. Plants can produce glucose through photosynthesis. It is widely used in confectionery manufacturing and medicine.
Chemical properties
It is the most widely distributed monosaccharide in nature. Glucose contains five hydroxyl groups, one aldehyde group, and has the properties of a polyhydric alcohol and an aldehyde.
Decomposes easily when heated under alkaline conditions. It should be kept tightly closed. It is quickly absorbed after oral administration and is used by tissues after entering the body. 1mol glucose releases 2870KJ energy after the body’s complete oxidation reaction.
Part of this energy is converted into 30 or 32mol ATP, and the rest of the energy is dissipated in the form of thermal energy to maintain human body temperature. It can also be converted into glycogen or fat by the liver or muscle.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
(1) The aldehyde group in the molecule is reducing and can react with silver ammonia solution
CH2OH (CHOH)4CHO + 2Ag (NH3)2OH— water bath heating → CH2OH (CHOH)4COONH4 + 2Ag ↓ + 3NH3 + H2O is oxidized to ammonium gluconate.
(2) The aldehyde group can also be reduced to hexadecanol.
(3) There are multiple hydroxyl groups in the molecule, which can be esterified with the acid.
(4) Glucose undergoes an oxidation reaction in the body and emits heat
(C6H12O6 + 6O2 (oxygen) + 6H2O == 6CO2 + 12H2O + energy).
(5) Glucose can be prepared by hydrolysis of starch under the catalysis of enzyme or sulfuric acid.
(6) Plant photosynthesis:
6CO2 + 6H2O (chlorophyll, sunlight catalysis)-C6H12O6 + 6O2.
(7) Reaction equation of glucose and fresh copper hydroxide:
CH2OH (CHOH).4 CHO + 2Cu (OH)2 – heating -> CH 2 OH (CHOH)4 of COOH + a Cu2 O↓ + 2H2O.
(8) Glucose is decomposed into water and carbon dioxide under certain conditions.
(9) Hydrolysis of maltose: C12H22O11 + H2O— catalyst → 2CH2OH (CHOH)4CHO (10) Hydrolysis of starch and cellulose: (C6H10O5)n + nH2O— catalyst → nCH2OH (CHOH)4CHO
Preparation of Glucose
1. A sugar aqueous solution obtained by partially hydrolyzing edible corn starch with food-grade acids and/or enzymes, purified and concentrated. Due to the different degree of hydrolysis, the amount of D-glucose contained can vary widely. Produced from corn starch, known as “corn syrup”. Glucose Chemical
2. Glucose can be prepared from starch by hydrolysis with hydrochloric acid or dilute Sulphuric acid. It can also be made from starch as raw material under the action of the starch saccharifying enzyme.
What is Glucose used for
(1) Fermentation Industry
The growth of microorganisms requires a suitable carbon-nitrogen ratio. Glucose is the carbon source of microorganisms. It is the main material of the fermentation medium.
For example, antibiotics, monosodium glutamate, vitamins, amino acids, organic acids, enzyme preparations, etc. require a large amount of glucose, and are also available. It is used as a raw material for microbial polysaccharides and organic solvents.
(2) Food industry
At present, crystalline glucose is mainly used in the food industry. With the improvement of living standards and the continuous development of science and technology in the food industry, the application of glucose in the food industry is more and more extensive, and the food industry is still the largest market for a long time to come.
(3) Chemical industry
Glucose is widely used in the industry. It is used as a reducing agent in the printing and dyeing industry. It is also commonly used as a reducing agent in the electroless silver plating industry such as the mirror industry, hot water bottle silver plating and glass fiber silver plating.
The application of glucose in the manufacture of chrome tanning agents in the tanning industry: chrome tanning agents are the best tanning agents for the manufacture of light leather (shoe leather, garment leather).
It has been 100 years since it was made with chromium salts. The leather has the characteristics of high shrinkage temperature, good elasticity, resistance to flexing, washing, and durability.
The chrome tanning agent is mainly basic chromium sulfate (basic chromium chloride can also be used, but the effect of the tanning agent is worse than that of the chromium sulfate).
The preparation method comprises the steps of reducing the dichromate to a basic chromium sulfate in a sulfuric acid solution by using glucose or sulfur dioxide as a reducing agent, thereby preparing a chrome tanning liquid, and concentrating and drying the mash to obtain a powdery chrome tanning agent. Glucose Chemical
(4) Synthesis and transformation
Glucose can be hydrogenated, oxidized, isomeric, alkaline degraded, esterified, acetalized, etc., synthesized or converted to other products.
For example, hydrogenated sorbitol; oxidized to glucuronic acid, diacid, etc., and further prepared into calcium acid, sodium, zinc acid and glucono delta lactone; isomerized to F42, F55, F90 fructose syrup and crystallized Fructose; can also be isomerized to mannose (manufacturing mannitol raw material), in which sorbitol can further produce vitamin C, which is widely used in clinical treatment, and mannitol 15% is safe and effective in reducing intracranial Compress drugs to treat brain edema and glaucoma.
