Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
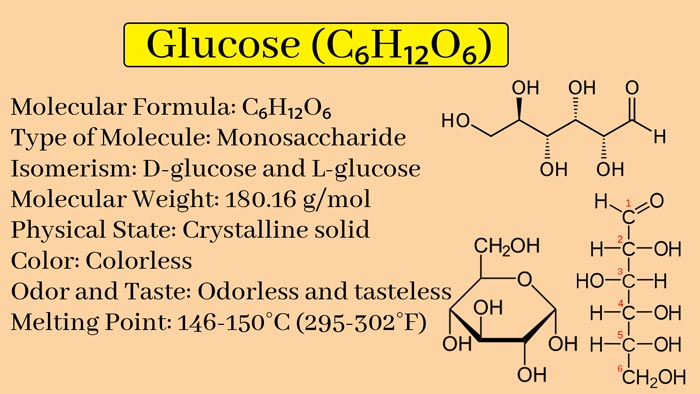
Glucose, organic compound, molecular formula C6H12O6. It is the most widely distributed and most important monosaccharide in nature, and it is a polyhydroxy aldehyde. Pure glucose is a colorless crystal with sweet taste but not as sweet as sucrose, easily soluble in water, slightly soluble in ethanol, and insoluble in ether. The natural glucose aqueous solution rotates to the right, so it belongs to ” dextrose “.
Glucose has an important status in the biological field, and it is the energy source and metabolic intermediate product of living cells, that is, the main energy-supplying substance of organisms. Plants can produce glucose through photosynthesis. It is widely used in confectionery manufacturing and medicine.
Glucose Function
Physical properties
Glucose is a colorless crystal or white crystalline or granular powder; odorless, sweet, hygroscopic, and easily soluble in water.
Optical rotation
The specific rotation of α-D-glucose at 20 degrees Celsius is +52.2.
Solubility
The maximum concentration of a single glucose solution is 50% at 20 degrees Celsius.
Sweetness
The specific sweetness of α-D-glucose was 0.7.
viscosity
The viscosity of glucose increases with increasing temperature.
Density: 1.544g / cm 3
Melting point: 153-158ºC
Boiling point: 410.797ºC at 760 mmHg
Flashpoint: 202.243ºC
Storage conditions: 2-8ºC
Chemical properties
It is the most widely distributed monosaccharide in nature. Glucose contains five hydroxyl groups, one aldehyde group, and has the properties of a polyhydric alcohol and an aldehyde.

Decomposes easily when heated under alkaline conditions. It should be kept tightly closed. It is quickly absorbed after oral administration and is used by tissues after entering the body. 1mol glucose releases 2870KJ energy after the body’s complete oxidation reaction.
Part of this energy is converted into 30 or 32mol ATP, and the rest of the energy is dissipated in the form of thermal energy to maintain human body temperature. It can also be converted into glycogen or fat by the liver or muscle.
Glucose Chemical Reaction
(1) The aldehyde group in the molecule is reducing and can react with the silver ammonia solution:
CH2OH(CHOH)4CHO + 2Ag(NH3)2OH— water bath heating → CH2OH(CHOH)4COONH4 + 2Ag↓ + 3NH3 + H2O is oxidized to ammonium gluconate.
(2) The aldehyde group can also be reduced to hexadecanol.
(3) There are multiple hydroxyl groups in the molecule, which can be esterified with the acid.
(4) Glucose undergoes an oxidation reaction in the body and emits heat
(C6H12O6 + 6O2 (oxygen) + 6H2O == 6CO2 + 12H2O + energy).
(5) Glucose can be prepared by hydrolysis of starch under the catalysis of an enzyme or sulfuric acid.
(6) Plant photosynthesis:
6CO2 + 6H2O (chlorophyll, sunlight catalysis)-C6H12O6 + 6O2.
(7) Reaction equation of glucose and fresh copper hydroxide:
CH2OH(CHOH)4CHO + 2Cu(OH)2 – heating -> CH2OH(CHOH)4 of COOH + aCu2O ↓ + 2H2O.
(8) Glucose is decomposed into water and carbon dioxide under certain conditions.
(9) Hydrolysis of maltose:
C12H22O11 + H2O— catalyst → 2CH2OH (CHOH)4CHO
(10) Hydrolysis of starch and cellulose:
(C6H10O5)n + nH2O— catalyst → nCH2OH(CHOH)4CHO
Preparation
1. A sugar aqueous solution obtained by partially hydrolyzing edible corn starch with food-grade acids and/or enzymes, purified and concentrated. Due to the different degree of hydrolysis, the amount of D-glucose contained can vary widely. Produced from corn starch, known as “corn syrup”.
2. Glucose can be prepared from starch by hydrolysis with hydrochloric acid or dilute sulfuric acid. It can also be made from starch as raw material under the action of a starch saccharifying enzyme.
Compound introduction
Basic Information
D-glucose; α-D-glucose; D-(+)-glucose; glucose syrup; corn glucose; maize sugar; glucose; 2, 3, 4, 5, 6-pentahydroxyhexanal
English name: α-D-glucose
English alias: DEXTROSE, alpha-D (+)-Glucose, alpha-D-glucose, α-D-Glucopyranose; D-Glucose-12C6; Dextrose; Cornsugar; Grapesugar; Blood sugar
Molecular formula: C6H12O6
Molecular weight: 180.15600
Exact mass: 180.06300
Security Information
Danger category code: R36 / 37/38
Safety instructions: S36 / 37 / 39-S26
Isomer
Isomers
Psicose, allulose; fructose; sorbose; tagalose; inositol
Chiral isomer
Allose, altrose, mannose, glucose, idose, galactose, talose
Optical isomers
α-D-glucofuranose, β-D-glucofuranose,
α-D-glucopyranose,
β-D-glucopyranose
Store
Under dry conditions, glucose has good stability, and the aqueous solution can be autoclaved. Overheating can cause the pH of the solution to decrease and caramelize.
Finished products in bulk should be stored in dry, low-temperature closed containers.
Chemical reaction
Verify aldehyde group
Glucose verification:
The glucose solution reacts with the fresh copper hydroxide suspension to form a brick-red precipitate. (Yellow precipitate is formed when the concentration is high)
Glucose Chemical Reaction
CH2OH(CHOH)4CHO + 2Cu(OH)2 → CH2OH(CHOH4COOH + Cu2O ↓ + 2H2O
Note 1)The newly prepared 2Cu(OH)2 suspension should be used with each other and should not be left for long.
2) When preparing freshly prepared Cu(OH)2 suspension, the NaOH solution must be used in excess.
3) The reaction solution must be heated directly to boiling.
4) Although glucose molecules contain aldehyde groups, d-glucose does not contain aldehyde groups.
Glucose solution and ammonia solution of silver reaction with a silver mirror reaction
CH2OH(CHOH)4CHO + 2Ag (NH3)2OH) → CH2OH(CHOH)4COONH4 + 2Ag↓ + 3NH3 + H2O
Note: 1) The inner wall of the test tube must be clean,
2) The silver ammonia solution can be used for a long time,
3) Water bath heating, not direct heating with an alcohol lamp,
4) Sodium hydroxide can be added to promote the reaction,
5) The silver mirror can be removed by dipping with dilute HNO3.
The silver produced by heating reduction adheres to the wall of the test tube and forms a silver mirror. Therefore, this reaction is also called a silver mirror reaction.
Physiological and biochemical
The central nervous system depends almost entirely on the supply of blood glucose as an energy source, and diabetes may occur once blood glucose rises to 80 mg%.
Industrially, glucose is produced by hydrolysis of starch. In the 1960s, microbial enzymes were used to produce glucose. This is a major innovation and has significant advantages over acid hydrolysis. In the production, the raw materials do not need to be refined, no acid and pressure-resistant equipment is needed, and the sugar solution has no bitter taste and high sugar yield.
Glucose is mainly used in medicine as a nutrient for injection (glucose injection).
In the food industry, glucose can produce fructose after isomerase treatment, especially fructose syrup containing 42% fructose, which has the same sweetness as sucrose and has become an important product in the current sugar industry. Glucose Chemical Reaction
Glucose is an indispensable nutrient for metabolism in the body. The heat released by its oxidation reaction is an important source of energy required for human life activities. It can be used directly in the food and pharmaceutical industries.
It is used as a reducing agent in the dyeing and tanning industry. Glucose is commonly used as a reducing agent in the mirror industry and the silver galvanizing process of thermos bottles. The industry also uses glucose as a raw material to synthesize vitamin C (ascorbic acid).
Metabolic function
Glucose is easily absorbed into the blood, so hospital staff, sports
enthusiasts, and ordinary people often use it as a powerful and fast
energy supplement.
Glucose strengthens memory, stimulates calcium
absorption and increases cell-to-cell communication. But too much can
increase insulin levels, leading to obesity and diabetes, too little can
cause hypoglycemia or worse, insulin shock (diabetic coma).
Glucose is important for brain function, and the metabolism of glucose is disturbed by the following factors: depression, bipolar disorder, anorexia and bulimia.
Patients with Alzheimer’s disease have recorded lower glucose concentrations than other brains with abnormal functions, causing strokes or other vascular diseases. Researchers have found that supplementing 75 grams of glucose in the diet increases memory test performance.
Glucose is absorbed into liver cells, which reduces the secretion of liver sugar, causing muscle and fat cells to increase glucose absorption. Excessive blood glucose is converted into fatty acids and triglycerides in the liver and adipose tissue.
Indication
Glucose has a wide range of clinical applications and is used for all kinds of water and calories required by patients with high fever, dehydration, coma or inability to eat.
When a large number of body fluids are lost in the body, such as vomiting and diarrhea, blood loss can be intravenously infused with 5% to 10% glucose and physiological saline to supplement water, salt and sugar, and used for hypoglycemia, drug poisoning.
The intravenous drip of 25% to 50% of hypertonic solution, due to its hypertonic effect, can make tissue dehydration and transient diuresis, combined with mannitol, alternately used to treat cerebral edema, pulmonary edema and reduce intraocular pressure.
Intravenous infusion of hypertonic glucose for hypoglycemia. Combined with insulin, because it can promote the transfer of potassium into cells, it is also one of the treatment measures for hyperkalemia.
Decomposition pathway
Natural glucose, whether free or combined, is in the D configuration. It mainly exists in the pyran configuration as the oxygen-containing ring in the aqueous solution.
Under normal temperature conditions, crystals can be precipitated from a supersaturated aqueous solution in the form of α-D-glucose hydrate (containing 1 water molecule), and the melting point is 80°C; while the crystals precipitated between 50-115°C are not Water α-D-glucose, melting point 146°C.
The stable form precipitated above 115℃ is β-D-glucose, and the melting point is 148 ~ 150℃. Glucose in the form of a furan ring exists only in a bound state in a few natural compounds.
D-glucose has the general chemical properties of aldose: under the action of oxidants, it produces gluconic acid, glucuronic acid or glucuronic acid; under the action of reducing agents, it produces sorbitol, under the action of weak bases, glucose can interact with the other two The six-carbon sugars with similar structures-fructose and mannose-are converted into each other by enol.
Glucose can also be combined with phenylhydrazine to form glucosamine, which is different from another glycocalyx in terms of crystal shape and melting point and can be used as a means of identifying glucose.
Most organisms have the ability of an enzyme system to break down D-glucose for energy. In living cells, such as mammalian muscle cells or single-cell yeast cells, glucose passes through the aerobic glycolytic pathway, the aerobic tricarboxylic acid cycle, and the biological oxidation process to generate carbon dioxide and water, releasing relatively Much of the energy is stored in the form of ATP (adenosine triphosphate) for life activities such as growth and exercise.
In the absence of oxygen, glucose is only decomposed to produce lactic acid or ethanol, and much less energy is released. Winemaking is an anaerobic decomposition process. Industrially, glucose produced by hydrolysis of starch with acid or enzyme can be used as raw material for food, wine, pharmaceutical and other industrial production.
Pharmacopoeia Standard
This product is D-(+)-glucopyranose monohydrate.
[Properties] This product is a colorless crystal or white crystalline or granular powder; odorless and sweet.
This product is soluble in water and slightly soluble in ethanol.
The specific rotation is about 10g of this product, accurately weighed, put in a 100ml measuring bottle, add an appropriate amount of water and 0.2ml of the ammonia test solution, after dissolving, dilute to the mark with water, shake well, leave it for 10 minutes, and measure at 25 ℃ according to the law (General Rule 0621), the specific rotation is + 52.6 ° to + 53.2 °.
[Identification] (1) Take about 0.2g of this product, add 5ml of water to dissolve it, and slowly drop it into the lukewarm alkaline tartrate test solution to form a red precipitate of cuprous oxide.
(2) Take an appropriate amount of this product under the item of loss on drying and measure it according to law. The infrared light absorption spectrum of this product should be consistent with the control spectrum (spectrum set 702).
[Check] Acidity Take 2.0g of this product, add 20ml of water to dissolve, add 3 drops of phenolphthalein indicator solution and 0.20ml of sodium hydroxide titration solution (0.02mol / L), it should show pink.
Clarity and color of the solution: Take 5.0g of this product. After dissolving in heated water, let cool and dilute to 10ml with water. The solution should be clear and colorless. If it is turbid, compare with No. 1 turbidity standard solution (General Rule 0902 first method), Must not be more concentrated, such as color development, and the control solution (take 3.0ml cobalt chloride solution for colorimetry, 3.0ml potassium dichromate solution for colorimetry and 6.0ml copper sulfate solution for colorimetry, diluted with water to 50ml) 1.0 Dilute to 10ml with water and not deeper.
Clarity of ethanol solution Take 1.0g of this product, add 20ml of ethanol, and place it on a water bath and heat to reflux for about 40 minutes. The solution should be clear.
Chloride Take 0.60g of this product and check it according to law (General Rule 0801). Compared with the control solution made from 6.0ml of standard sodium chloride solution, it must not be more concentrated (0.01%).
Sulfate Take 2.0g of this product and check it according to the law (General Rule 0802). Compared with the control solution made from 2.0ml of standard potassium sulfate solution, it must not be more concentrated (0.01%).
Sulfite and soluble starch Take 1.0g of this product, add 10ml of water to dissolve, add 1 drop of the iodine test solution, it should be yellow immediately.
Loss on drying Take this product and dry to constant weight at 105 ° C. Lose weight is 7.5% ~ 9.5% (General rule 0831).
Ignition residue shall not exceed 0.1% (General Rule 0841).
Protein Take 1.0g of this product, add 10ml of water to dissolve, add 3ml of sulfosalicylic acid solution (1 → 5), no precipitation should occur.
Barium salt Take 2.0g of this product, add 20ml of water to dissolve, the solution is divided into two equal parts, one part is added with dilute sulfuric acid 1ml, the other part is added with 1ml water, shake well, and left for 15 minutes, both liquids should be clarified.
Take 1.0g of calcium salt, add 10ml of water to dissolve, add 1ml of the ammonia test solution and 5ml of ammonium oxalate test solution, shake well and let stand for 1 hour, if turbidity occurs, and standard calcium solution [precisely weigh 0.1250g of calcium carbonate, set In a 500ml measuring flask, add 5ml of water and 0.5ml of hydrochloric acid to dissolve, dilute to the mark with water, and shake well. Each 1ml is equivalent to 0.1mg of calcium (Ca)] 1.0ml comparison solution made of must not be more concentrated (0.01%).
Take 2.0g of iron salt, add 20ml of water to dissolve, add 3 drops of nitric acid, slowly boil for 5 minutes, let cool, dilute with water to make 45ml, add 3.0ml of ammonium thiocyanate solution (30 → 100), shake well, such as Compared with the control solution made by the same method with 2.0ml of the standard iron solution, the color must not be deeper (0.001%).
Heavy metal Take 4.0g of this product, add 23ml of water to dissolve, add 2ml of acetate buffer solution (pH 3.5), and check according to the law (general rule 0821 first method), containing no more than five parts per million of heavy metals.
Take 2.0g of arsenic salt, add 5ml of water to dissolve, add 5ml of dilute sulfuric acid and 0.5ml of potassium bromide bromine test solution, heat in a water bath for about 20 minutes to keep a slight excess of bromine, and add bromine if necessary.
An appropriate amount of potassium bromide test solution, and replenish evapotranspiration at any time, let cool, add 5ml of hydrochloric acid and the appropriate amount of water to 28ml, check according to the law (General Law 0822 first method), should meet the requirements (0.0001%).
Microbial limit Take 10g of this product and make a 1:10 test solution with sterile sodium chloride-peptone buffer pH 7.0.
The total number of aerobic bacteria, molds and yeasts shall be 1ml for the test solution, and shall be checked according to law (General 1105 plate method). The total number of aerobic bacteria in 1g of the test product shall not exceed 1,000cfu, and the total number of molds and yeast shall not exceed 100cfu.
Take 10ml of the test solution of 1:10 E. coli and check it according to law (General Rule 1106). 1g of the test solution shall not be detected.
[ Category ] Nutritional medicine.
[ Storage ] Keep sealed.