Glycerol: What Glycerol used for? | Preparation and Properties
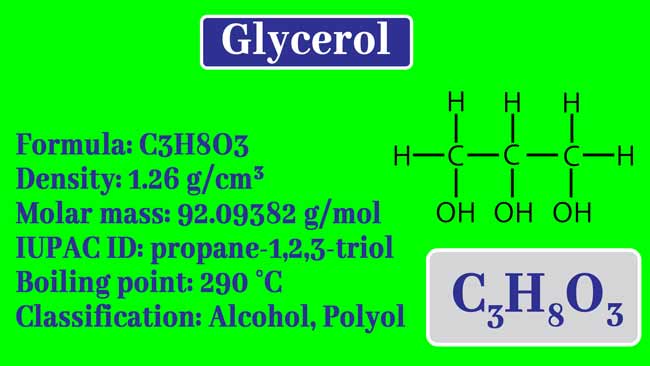
It is a Tri-hydric alcohols. Its formula is C3H8O3. By substituting one hydrogen atom containing one carbon atom in each molecule of propane by an -OH group, its structure formula is obtained.
Its structure formula is the following.

Its name is 1,2,3-propanetriol in the IUPAC method. In nature it is found as an ester with mono carboxylic acid present in oils and fats. The ester made with its high monocarboxylic acid is called Glycerides.
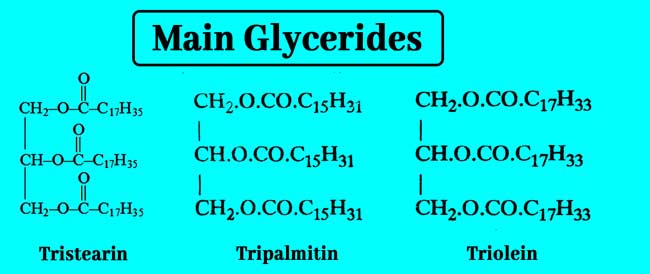
Following are the formulas and names of some of the major Glycerides.
Glycerol Preparation
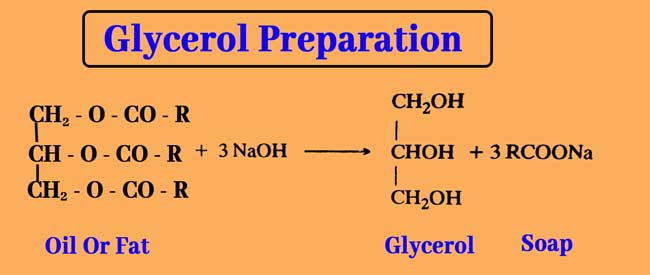
Glycerol can be obtained from water decomposition of oils and fats in commercial quantities. Acidic water decomposition of oils and fats provides glycerol and high mono carboxylic acid.
This reaction is used in the candle industry and the industrial manufacture of glycerol. The alkaline water decomposition of fats and fats produces sodium or potassium salt of glycerol and mono carboxylic acid.
Those are called Soaps. This reaction is used in the soap industry and the industrial manufacture of glycerol.
Physical Properties of Glycerol
Glycerol is a colorless, odorless, and thick liquid. It tastes sweet. Its boiling point is 290°C. But on heating it starts decomposing before this temperature. It is miscible in ethanol and water. But is immiscible in ether. On cooling it forms transparent crystal whose melting point is 180°C. It is heavier than water. Its relative density is 1.265.
Chemical Properties of Glycerol
There are two types of Hydroxyl group in glycerol. Two primary and one secondary -OH group.
Hence, it exhibits properties of both these types of hydroxyl group. Its main chemical reactions are as follows.
- Glycerol: What Glycerol used for? | Preparation and Properties
- Formic Acid: Preparation, Properties, Uses and Tests
- Chloroform: Preparation, Properties and Uses
- Diethyl Ether: How Diethyl Ether is prepared | Uses | Properties
- Ethyl Alcohol: Are ethyl alcohol and ethanol the same?
- Methanol(CH3OH): Properties, Preparation, uses and Tests
- IUPAC Name : How to find the IUPAC name of compounds.
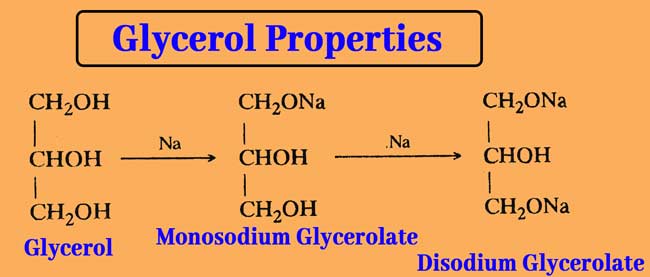
Reaction with Sodium or Potassium: Reaction with sodium or potassium replaces the hydrogen atoms of the primary -OH group. The reaction results in the formation of mono and di glycerolate. Na or K have no effect on the secondary alcohol group.
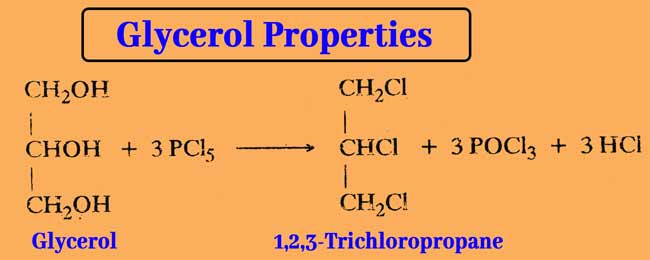
Reaction with phosphorus pentachloride: PCl5 reacts with glycerol and displaces three of its -OH group with chlorine atoms.
Reaction with mono oxalic acids: The reaction of glycerol with mono oxalic acids results in the formation of mono, di and tri ester.
Example: Glycerol mono, di, and tri acetate are obtained with acetic acid respectively.

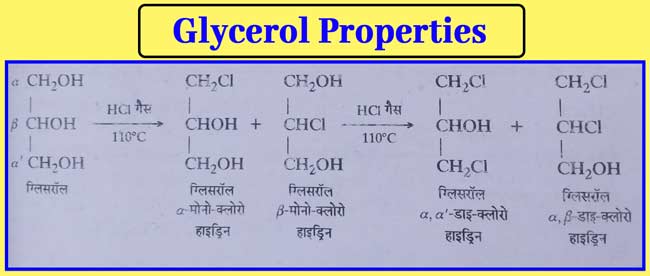
Reaction with HCl gas: α- and β- monochlorohydrin are
formed when the theoretical amount of HCl gas is flowed in glycerol at
110°C heat. After this, α,α-di-chlorohydrin and α,β-di-chlorohydrin are
formed when about 25% more than the theoretical amount of HCl gas flows
at this temperature.
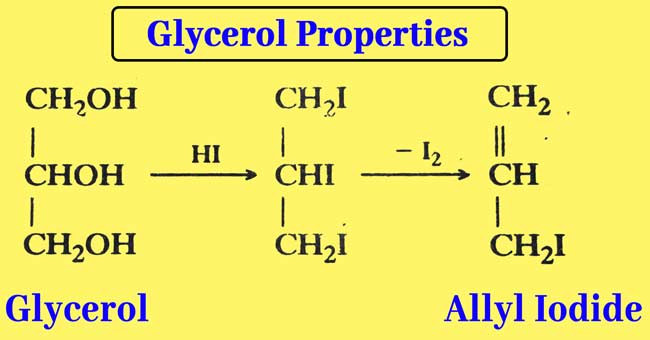
Reaction with Hydroiodic acid: Glycerol is reacted with a small amount of HI when allyl iodide is obtained.
When an overdose of HI is used, ellyl iodide is formed first but finally isopropyl iodide is obtained.
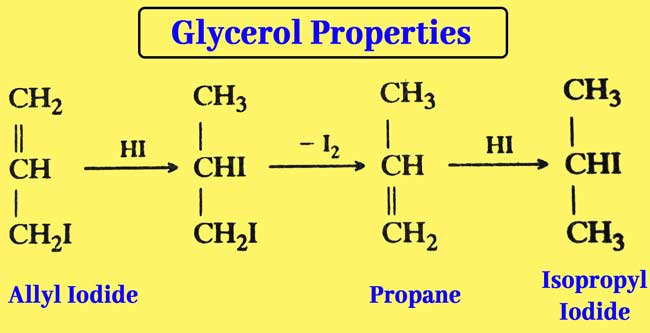
The reaction of glycerol with a mixture of PI3 or red phosphorus and iodine is also similar and isopropyl iodide is obtained as a result of the reaction.
Reaction with Nitric Acid: The addition of glycerol to a cold mixture of concentrated sulfuric acid and concentrated nitric acid produces an explosive substance called Glyceryl Trinitrate. Also called Nitroglycerin.

Nitroglycerin is a toxic and oily liquid. It is an important explosive used in making dynamite. It was discovered by Alfred Nobel. It is also called noble’s oil. This reaction is carried out at a temperature less than 20°C, otherwise an explosion occurs.
Reaction with oxalic acid: Glycerol is reacted with oxalic acid in two ways.
Formic acid is formed by heating glycerol with oxalic acid to 100°- 110°C.
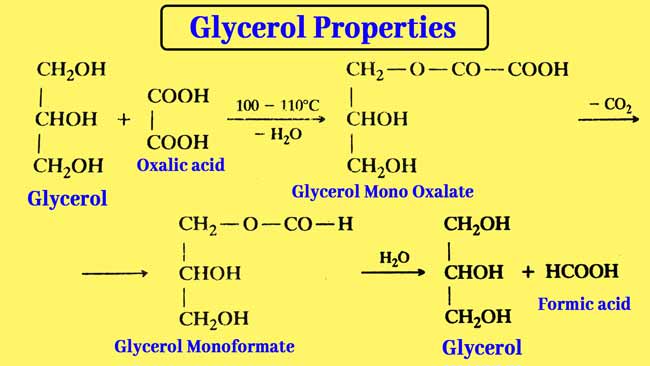
Allyl alcohol is obtained by heating glycerol with oxalic acid at 260°C.

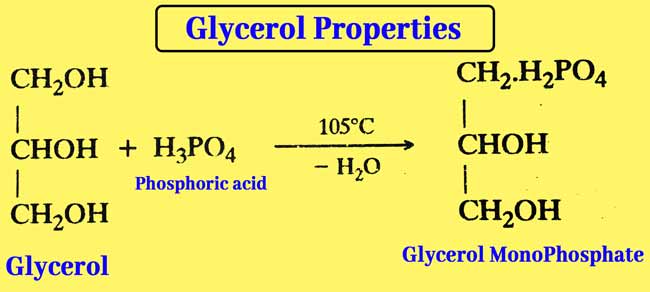
Reaction with phosphoric acid: The reaction of glycerol and phosphorus acid at 105°C results in Glycerol Mono Phosphate.
Dehydration: It is dehydrated when glycerol is heated with potassium hydrogen sulphate and the deodorant substance acrolein is obtained.

Oxidation: The oxidation of glycerol provides various substances depending on the nature of the oxidant.
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
- DDT full Form || What was DDT used for? || Is DDT harmful to humans?
- Reproduction in Organisms
- Chemicals in Medicine: Class 12
- What is Urea || How to make Urea Fertilizer, || Urea uses
Example:
Glyceric acid and tartronic acid are obtained upon oxidation by dilute HNO3.
Glyceric acid is mainly obtained by oxidation by concentrated nitric acid.
The mesoxalic acid is obtained mainly by oxidation by bismuth nitrate.
Glycerol aldehyde and dihydroxyacetone are obtained upon oxidation by bromine water, sodium hypobromite or Fenton’s reagent (FeSO4 + H2O2).
Formaldehyde and formic acid are obtained by oxidation by par iodic acid(HIO4).
Oxalic acid water and CO2 are formed upon oxidation by KMnO4. The mixture of glycerol and KMnO4 is explosive. This mixture is used to make time bum.

Glycerol Uses
The major use of glycerol is in making nitroglycerine. Nitroglycerine is used to make explosive called dynamite and propellant called cordite.
Glycerol is useful in keeping the raid ink, shoe polished, and typing machine moist.
Glycerol is used in cosmetics and transparent soap making.
Used as a lubricant in watches.
Many important organic compounds such as formic acid and allyl alcohol are used to make.
It is used to make acid resistant cement.
It is used as glycerophosphate in protein tonic.
It is used as a preservative to keep food and fruits safe.
It is used in making ointments that cause inflammation etc.
Glycerol Tests
On heating glycerol with potassium hydrogen sulfate, a deodorant, acrolein is formed which reduces the Fehling solution and ammonium silver nitrate solution.
Dunsten’s test: Pink colour is obtained by adding phenolphthalein drops in 1% solution of Icing. On adding glycerol to this solution, the pink color flies away. The pink color reappears on heating the solution and disappears again on cooling.
These reagents are not degraded by heating glycerol with Fehling solution or ammonium silver nitrate solutions.