What is heavy water used for?
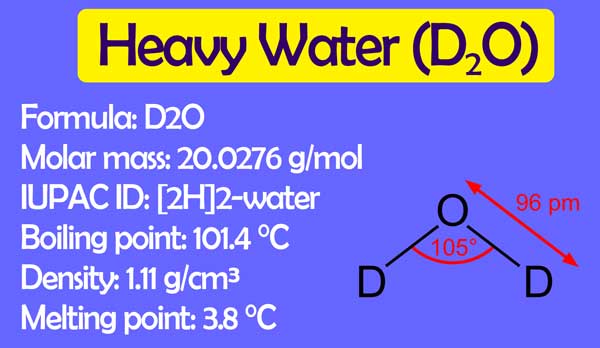
The oxide (D2O) of Deuterium is also called heavy water. About 6,000 parts of ordinary hydrogen contain about 1 part deuterium.
Harold Urey was discovered Heavy Water in 1931. For the discovery of Deuterium, Harold Urey received the Nobel Prize in 1934. It was first made by Macdonald in 1933 in the concentrate by the simple decomposition of ordinary water.
 Preparation of Heavy Water
Preparation of Heavy Water
Heavy water is obtained by burning deuterium in air.
2D2O → 2D2 + O2
Deuterium is also obtained by an exchange reaction with ordinary water.
H2O + D2 – 2000C/Pt → 2D2O + H2
Fractional distillation of water:
It can be separated from ordinary water by fractional distillation of ordinary water. For this, a fractionating column about 1200 cm long is used.
The boiling point of heavy water is higher than the boiling point of ordinary water. Therefore, light water (H2O), distillate, and heavy water are obtained from a residual distillate of ordinary water in the residue.
Electrolysis method:
Heavy water can also be separated from ordinary water by electrolysis of ordinary water. Uery found here that electrolysis of H2O occurs about 6 times more quickly than electrolysis of it when electrolysis of ordinary water. Therefore, upon electrolysis of ordinary water, the ratio of heavy water in it increases gradually.
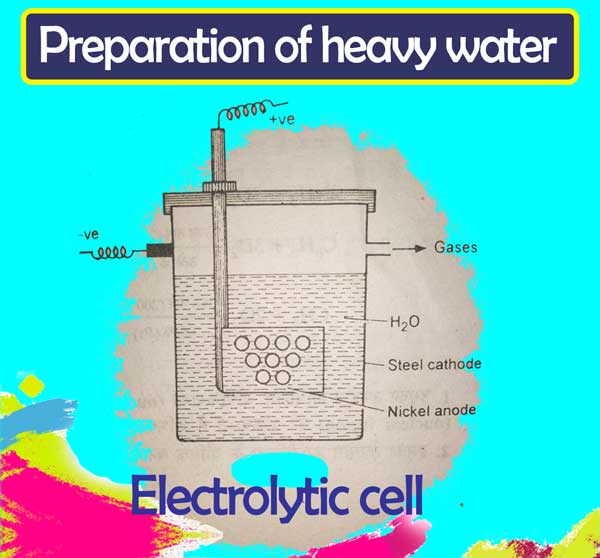
There are several methods of obtaining it by the electrolysis of ordinary water. In these, the method developed by Taylor, Eyring & Frost is nowadays used for heavy water industrial manufacture.
An iron vessel in the electrolytic cell used in this method acts as a cathode and the anode is made of nickel. The first is the decomposition of the N/2 solution in simple water of NaOH.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
When the volume of water remains about one-fourth of the initial volume, it is distilled and mixed with NaOH in the distillate and then its concentration is again N/2. And then do the electrical decomposition.
Physical Properties
Like ordinary water, It is also colorless, odorless, and free liquid. There is a difference between physical constants of this and light water.
The boiling point and melting point of light water are 1000C and 00C while the boiling point and melting point of heavy water are 101.420C and 3.80C, but its density is 1.11 gram per cubic cm which is 11% more than the density of light water at this temperature.
The maximum density of light water is at 40C. While the maximum density of it is at 110C. Its Molecular mass is 20.
Chemical properties:
In chemical properties, it is similar to light water but the speed of chemical reactions of it is half the speed of chemical reactions of light water.
Hence some of the main chemical properties are as follows.
Electrolyte Process
In the presence of a small amount of legitimate electrolyte, the following reaction occurs when it is made a legitimate electrolyte.
2D2O → 2D2 + O2
It forms deuterium gas by reacting with sodium and other functional metals.
2Na + 2D2O – 3500C → 2NaOD + D2
Action with oxide:
It reacts with acid oxides produce acids and reacts with Alkaline Oxides it makes alkali.
Na2O + D2O → 2NaOD
N2O5 + D2O → 2DNO3
SO3 + D2O → D2SO4
By reacting with nitrides, it causes heavy ammonia.
Mg3N2 + 6D2O → 3Mg(OD)2 + 2ND3
It reacts with carbides to form deuterium-containing hydrocarbon.
Al4C3 + 12D2O → 4Al(OD)3 + 3CD4
CaC2 + 2D2O → Ca(OD)2 + C2D2
Exchange reactions:
Hydrogen and deuterium exchange when hydrogen-containing compounds react with heavy water. Disposable hydrogen present in hydrogen-containing compounds i.e. hydrogen which has partial or full positive charge easily displaced by deuterium.
The exchange of unregulated hydrogen and deuterium takes place at a very slow rate. And this action requires a catalyst.
HCl + D2O → DCl + HOD
NaOH + D2O → NaOD + HOD
NH4Cl + 4D2O → ND4Cl + 4HOD
Biological Properties:
Is it safe to drink heavy water?: The small amount of heavy water present in natural water is not harmful to live organisms. But pure or concentrated heavy water is harmful to live organisms. Many living beings die using pure heavy water. And the growth of many organisms stops.
Heavy water reactions are slower than ordinary water reactions. For this reason, the use of heavy water makes biochemical reactions unbalanced. And it is harmful to live beings.
Use of Heavy Water:
In Nuclear Reactors, it is used as a moderator.
It is used to make compounds of deuterium and deuterium.
It is also used to study the processes of various reactions and processes.
