Helium is a rare gas. The element name is derived from Greek, meaning “sun”. In 1868, Jansson of France used a spectroscope to observe the surface of the sun and found a new yellow spectral line, which was considered to belong to an unknown element on the sun, hence the name He.
Helium is usually a colorless, odorless gas, not only at normal atmospheric pressure at the curing material. Helium is the least active element. The application of helium is mainly as a protective gas, a working fluid for an air-cooled nuclear reactor, and a cryogenic cryogen.
In addition, because it is less dense than air and stable in nature, helium can also be used as a floating gas.
Content distribution
Helium is present in the entire universe, accounting for 23% by mass, second only to hydrogen. However, it is mainly found in natural gas or radioactive ore in nature. In the Earth’s atmosphere, the concentration of helium is very low, only one-fifth of 52,000.
The helium contained in radioactive minerals on Earth is the product of alpha decay. Helium in certain natural gas contains economically worth extracting, up to 7%.
In the United States, about 1% of helium, and 4.6 cubic centimeters of helium per cubic meter of air in the surface, accounting for about the entire volume is 0.0005% and the density is only 7.2 times that of air. It is the smallest density gas except for hydrogen.
| Crust content | 0.008 (ppm) |
| Elements in the sun | 230000 (ppm) |
| Elements in seawater | 0.000006 (ppm) |
Helium on Earth is mainly the product of the decay of radioactive elements, and alpha particles are the nucleus of helium. In industry, it can be extracted from natural gas containing helium up to 7%. It can also be obtained from helium-neon mixed gas by fractional distillation in liquid air.
Physical properties
Basic Information
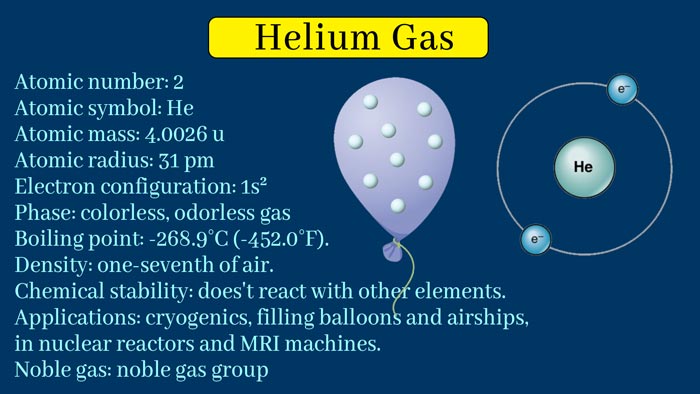
The element symbol He, atomic number 2, atomic weight 4.002602 (helium 4), is a kind of rare gas. The element name is derived from Greek, and the original meaning is “sun”. helium uses
There are two natural isotopes of helium: helium 3 and helium 4. The helium present in nature is basically helium 4. The relative atomic mass is 4.003. In 1868, someone used a spectroscope to observe the surface of the sun and found a new yellow spectral line, which was considered to belong to an unknown element on the sun, hence the name He. The helium content in the air is 0.0005%.
Helium is a colorless and odorless gas under normal conditions; melting point -272.2 ° C (25 atmospheres), boiling point -268.9 ° C; density 0.1785 g / l, critical temperature -267.8 ° C, critical pressure 2.26 atmospheres; solubility in water 8.61 cm³ / Kg of water.
Helium is one of the inert elements, its molecular formula is He, it is a rare gas, colorless, odorless and tasteless. Its solubility in water is the smallest of the known gases, and it is also the least dense gas except for hydrogen. The density is 0.17847 g / l, and the melting point is -272.2 ° C (25 atmospheres ).
The boiling point is -268.9 ° C. It is the most difficult gas to liquefy, and its critical temperature is -267.9 ° C. The critical pressure is 2.25 atmospheres. When the temperature drops below -270.98 ℃ after liquefaction, it has a small surface tension, strong thermal conductivity, and hardly shows any viscosity. Liquid helium can be used to get low temperatures near absolute zero (-273.15 ° C). The chemical properties are very inert and neither burn nor support combustion. Helium is also the most difficult gas to liquefy. helium uses
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
Helium is usually a colorless, odorless gas. It is the only substance that cannot be cured at standard atmospheric pressure. When the temperature of liquid helium drops to 2.18K, its properties change suddenly, and it becomes a superfluid, which can flow upward along the container wall. The thermal conductivity is 800 times that of copper, and it becomes a superconductor; its specific heat capacity, surface tension, and compressibility are all unusual.
Due to the extremely low temperature of liquid helium, many wonderful physical phenomena occur at this temperature. Many important physical experiments are performed at low temperatures. Physicists from all over the world are studying liquid helium, hoping to reach a lower temperature through liquid helium and to study what kinds of substances will change at low temperatures, and what properties we do not know. This has created a new branch of physics-low temperature physics.
| Melting point | -272.2 ℃ (25 atmospheres); |
| Boiling point | -268.9 ℃; |
| density | 0.1785 g / l |
| Critical temperature | -267.8 ℃ |
| Critical pressure | 2.26 atm |
| Solubility in water | 8.61 cubic centimeters per kilogram of water |
| Thermal conductivity | 151.3W / (m · K) |
| Crystal structure | Unit cell |
Helium-4
The following table shows some basic physical properties of liquid helium (helium 4) (the status of some parameters is unknown):
| Normal boiling point / K | 4.224 |
| Density / kg / m³ | 124 96 |
| Evaporation heat / kJ / kg | 20.73 |
| Specific heat / kJ / (kg · K) | 4.56 |
| Viscosity / MPa · s | 3.57 |
| Thermal conductivity / mW / (m · K) | 2.72 |
| Dielectric constant | 1.0492 |
| Critical temperature / K | 5.201 |
| Critical pressure / MPa | 0.227 |
Helium-3
Helium 3 is a stable isotope of helium in nature, with an atomic weight of 3.016. The nucleus consists of two protons and one neutron. Under normal circumstances, helium 3 is a colorless, odorless, non-toxic, non-combustible inert gas, and its density is 0.1345 kg / m 3 at 0 ° C and 0.101325 MPa.
The following table shows some basic physical properties of liquid helium (helium 3): helium uses
| Normal boiling point / K | 3.191 |
| Density / kg / m³ | 82.3 |
| Evaporation heat / J / mol | 20.56 |
| Specific heat at 1.0K / J / (mol · K) | 4.222 |
| Viscosity at 3.2K / mPa · s | 3.57 |
| Thermal conductivity at 3.2K / mW / (m · K) | 20 |
| Critical temperature / K | 3.324 |
| Critical pressure / MPa | 0.115 |
Super fluidity
Carmelin Onez was the first scientist to get liquid helium. He lowered the temperature further and tried to obtain solid helium without success (solid helium was first obtained by Kisom in 1926 by reducing the temperature and increasing the pressure).
For general liquids, the density will gradually increase as the temperature decreases. Carmelin Onez lowered the temperature of the liquid helium and increased the density of the liquid helium. However, when the temperature dropped to minus 271 ° C, the liquid helium suddenly stopped bubbling and the density also suddenly decreased.
This is another liquid helium. Camerin Ones called the former bubbling liquid helium helium I and the latter stationary liquid helium helium Ⅱ.
Press a small glass into helium II. The glass was gradually filled from empty. Lift up this small glass filled with liquid helium. When hanging in midair, liquid helium appears under the glass. After a while, the liquid helium in the glass “leaks”.
Helium II can flow backward, and it will flow back up along the wall of the glass. This phenomenon can only occur at low temperatures. It is called ” superfluidity “, and helium II with “superfluidity” is called superfluid.
Later, many scientists studied this strange phenomenon and made many new discoveries. For example, the Helium Knife Fountain discovered by Alan and others in 1938. In a glass tube, there is very fine corundum, and a fine nozzle is connected to the upper end. The glass tube is immersed in helium II, and the thick lower part of the glass tube is illuminated, and the thin nozzle will spray out the helium Ⅱ fountain. The stronger the light, the higher the spray, which can reach several centimeters.
Helium II fountains are also special properties of superfluids. In this experiment, light energy is directly converted into mechanical energy.
Superconducting phenomenon
At the temperature of liquid helium, a lead ball is placed on a lead ring. Shot put will float on the ring as if weightless, keeping a certain distance from the ring. At the same temperature, tie the magnet with a thin chain and slowly place it on a metal plate. When the magnet is about to hit the plate, you can observe that the chain is loose and the magnet floats on the plate. If you tap the magnet lightly at this time, it will rotate by itself. This phenomenon can only be observed at low temperatures and does not occur at high temperatures.
This is a superconducting phenomenon at low temperatures. In some metals, the movement of the nucleus almost stops at the temperature of liquid helium, and the resistance to electrons becomes extremely small, so the resistance will disappear and become a superconductor since the magnetic field lines cannot pass through the superconductor, it is formed between the superconductor and the magnet With a large magnetic field, the repulsive force of the magnetic field supports the shot and the magnet, causing them to float in midair. This is the Meissner effect (Meissner Effect), this effect can be used to manufacture a magnetic levitation train.
Chemical properties
Helium is the least active of all elements and it is extremely difficult to form compounds because the distance from the nucleus to the electron layer of helium is very small and a stable structure is achieved. Its nature determines its use. The application of helium is mainly as a protective gas, a working fluid for an air-cooled nuclear reactor, and an ultra-low temperature refrigerant.
On February 6, 2017, the team of Wang Huitian, Zhou Xiangfeng and their collaborators from Nankai University in China published a paper on the synthesis of sodium helium compound-Na2He under high pressure in Nature Chemistry, ending helium The history of element-free compounds indicates that China has reached the forefront in the field of rare gas chemistry.
Conjecture on fluoride production
Pimental et al. Proposed three methods for preparing HeF₂ by nuclear transformation based on the electron arrangement of HeF₂ similar to the stable HF₂⁻.
1. Tritium beta decay method
Tritium should change to helium after beta decay. In this way, after the decay of the beta compound, it may become a helium compound. In order to facilitate the reaction, the deuterium and hydrogen isotopes are first returned to fix the deuterium in the solid lattice of KHF₂. After the nuclear reaction of TF₂⁻ trapped in the crystal lattice, HeF₂ is formed.
TF₂⁻ → HeF₂ + β⁻
The recoil energy of deuterium during the decay process will not cause the newly generated helium difluoride to break the chain. The half-life of deuterium decay is 12.25 years. It is estimated that ¹⁰Ci’s plutonium can only produce 10 μmol of HeF₂ after 4 to 5 months.
2. Thermal neutron irradiation method
LiF is irradiated with thermal neutrons to produce a nuclear reaction
₃⁶Li + ₀¹n → ₂⁴He + ¹³T
After Li (n, α) reaction, the generated helium nuclei are combined with F – in the parent lattice to form HeF₂.
3. Directly bombard solid fluorine with alpha particles to prepare HeF₂
From this point of view, of the three methods, the first method is most likely to make HeF₂, but no report has been seen so far. thought that although the electron arrangement of HeF₂ and HF₂⁻ is similar, HF₂⁻ is a compound where H⁻ interacts with two F atoms to generate compounds. It is doubtful whether HeF₂ exists.
Ionic compounds
Helium hydride ion, with the chemical formula HeH, is a positively charged ion. It was first discovered in 1925 and was made by reacting protons and helium atoms in the gas phase. It is the strongest known acid with a proton affinity of 177.8 kJ / mol. This ion is also called helium-hydrogen molecular ion.
Some people think that this kind of material can exist in natural interstellar matter. This is the simplest heteronuclear ion, which can be compared with the co-nuclear hydrogen molecular ion H₂. Unlike H₂, it has a permanent bond dipole moment, making it easier to exhibit spectral characteristics.
HeH⁺ cannot be prepared in the condensed phase, because it will cause it to interact with any anion, molecule, atom. However, it is possible to predict its acidity in aqueous solution using Gas’s Law.
Ionization -360 kJ / mol of the free energy change corresponding to P K A -63.
HeH in covalent bond lengths is 0.772 Å.
Other helium-hydrogen ions are known or studied in theory. HeH₂, which has been observed by microwave spectroscopy, scientists have calculated that its affinity energy is 6 kcal / mol, while HeH₃ is 0.1 kcal / mol.
Neutral molecule
Unlike helium hydride ions, hydrogen and helium neutral molecules are generally unstable. However, it is stable as an excimer in the excited state and was first observed in the spectrum in the mid-1980s.
pka: -63 (presumably), much stronger than fluoroantimonate.
Even so, these ions or molecules appear only “instantaneously” or are calculated only, so they are difficult to consider as “compounds”.
Helium sodium compounds
On February 6, 2017, the team of Wang Huitian, Zhou Xiangfeng and their collaborators from Nankai University in China published a paper on the synthesis of sodium helium compound-Na 2 He under high pressure in Nature Chemistry, ending The history of helium-free compounds marks that China has reached the forefront in the field of rare gas chemistry.
Previously, researchers have found ways to pair other elements with helium. But for a long time, nothing has been formed that can stably exist. The most common example is the van der Waals force of helium and other elements, which can exist without covalent or ionic bonds. At extremely low temperatures, helium can indeed form Van der Waals forces, but it is extremely weak and cannot be maintained for long.
Helium’s strong stabilizing force comes from its closed-shell electronic configuration, its shell is in a complete state, and there is no space to combine with other atoms through shared electrons. But this is the case in the surface environment of the earth.
As the second most abundant element in the universe, helium plays an important role in the formation of stars and giant gas planets. In extreme conditions in outer space or deep on the earth, it may follow unusual rules. Now researchers have just verified this bizarre phenomenon.
“Extremely high pressures, such as in the core of the earth or other giant stars, can completely change the chemical properties of helium,” said Alex Boldyrev, co-author of the article at Utah State University.
Researchers performed calculations through a “crystal structure prediction” model and found that under extreme pressure, a stable helium-sodium compound can form. Then they really created a compound never seen in the diamond cavity experiments: Na2He. Experiments can provide conditions equivalent to 1.1 million times the Earth’s atmospheric pressure for helium and sodium atoms.
This result was too unexpected, so it encountered huge difficulties when it was published, and researchers spent more than two years persuading reviewers and editors.
Based on these results, the research team predicts that if the pressure reaches 10 million times their experimental level, sodium will easily react with helium to produce stable Na 2 He. Even more amazing is that the composition of this compound does not require any chemical bonds.
Professor Wang Huitian of Nankai University is the co-corresponding author of the study. According to him: “The compounds found are very strange: Helium atoms usually do not form any chemical bonds, and the existence of new substances fundamentally changes the chemical interaction between sodium atoms Effect, forcing electrons to concentrate in the cubic space of the structure, while having insulation capabilities. “
The crystal structure of Na2He is a region in which sodium (purple) and helium (green) atoms alternate, and a common electron (red) exist.
“This is not really a chemical bond,” Popov said, “but helium can make this structure stable. If you remove helium atoms, the structure will not be stable.”
The following are other expressions of the compound. The pink in the left is sodium and the white is helium. In the right, sodium and helium form a cube, and the red dots are electrons.
Sublattice analysis showed that He’s occupancy caused electrons to be localized into the atomic gaps and form polycentric bonds under the gravitational pull of the Na nucleus, thus the whole system became an electronic salt system.
In this process, the isolated electrons, the inner electrons of Na and the inner 1s electrons of Him and the outer 2s, 2p orbitals strongly overlap. Affected by Pauli’s incompatibility principle, the 1s electron density of He and the distribution of outer electron orbitals were forced to change, which resulted in He obtaining 0.15 electrons during the formation of Na 2 He.
This work confirms that He has weak chemical activity under high pressure and can form compounds with Na, which has significantly enhanced reducibility under high pressure.
Although the recent breakthrough research on metal hydrogen has encountered great doubts, the data in this article is much more solid. Physicist Henry Rzepa, from Imperial College London, compared this study with the discovery of metal hydrogen, saying: “This is more reliable science, and helium compounds are a major breakthrough.”
Isotope
There are eight known helium isotopes, including helium 3, helium 4, helium 5, helium 6, helium 8, etc., but only helium 3 and helium 4 are stable, and the rest are radioactive. In nature, helium 4 accounts for the largest proportion of helium isotopes, mostly from alpha decay of other radioactive materials, which releases helium 4 nuclei. On Earth, helium 3 content is extremely small, and they are all produced by the beta decay of super heavy hydrogen (rhenium).
Helium-2: Its nucleus has only 2 protons, so far it is only a hypothetical particle, but if the strong nuclear force is increased by 2%, it may exist.
Helium-5 is one of the isotopes of helium, and the element symbol is He. Its nucleus consists of two protons and three neutrons. It is radioactive and emits neutrons with a half-life of 0.6 MeV.
Helium-6: The nucleus contains 2 protons and 4 neutrons and is very unstable.
Helium-7: The nucleus contains 2 protons and 5 neutrons. It decays into helium-6 and is very unstable.
Helium-8: The nucleus contains 2 protons and 6 neutrons and is very unstable.
Helium-9: The nucleus contains 2 protons and 7 neutrons and is very unstable.
Helium-10: The nucleus contains 2 protons and 8 neutrons and is very unstable.
Preparation method
Natural gas separation method: In industry, mainly helium-containing natural gas is used as a raw material, liquefaction fractionation is repeatedly performed, and then activated carbon is used for adsorption purification to obtain pure helium. Ammonia Method: In the synthesis of ammonia, the tail gas from the separation and purification of helium available.
Air fractionation: air from the liquid by fractionation helium neon gas mixture.
Uranium ore method: uranium ore containing helium is roasted to separate gas, and then chemical methods are used to remove impurities such as water vapor, hydrogen and carbon dioxide to purify helium.
Function use
Because helium is light and non-flammable, it can be used to fill airships, balloons, thermometers, tubes, diving suits, etc. It can also be used as protective gas in atomic reactors and accelerators, lasers, rockets, smelting and welding. It can also be used to fill bulbs and neon tubes. It is also used to make foam plastics.
Due to the low solubility of helium in the blood, it can be added to oxygen to prevent decompression sickness, as a breathing gas for divers, or to treat asthma and suffocation.
The temperature of liquid helium (-268.93 ° C) is close to absolute zero (-273 ° C), so it is used as a superfluid in superconducting research to make superconducting materials. Liquid helium is also commonly used as a coolant and refrigerant. In medicine, it is used in argon-helium knives to treat cancer. It can also be used as part of artificial atmospheres and laser media.