Hydrogen Chloride : Preparation, Properties and Uses
In 1648, Glouber obtained Hydrogen Chloride Gas by heating sodium chloride (common salt) and concentrated sulfuric acid. Debi proved in 1810 that it is a compound of hydrogen and chlorine.
Hence it was named Hydrochloric Acid. It is also called acid of salt. The aqueous solution of hydrogen chloride gas is called hydrochloric acid.
Occurrence and Availability
It is present in various gases emanating from the eruption of volcanic mountains. It is found in 0.2 to 0.4 percent quantity in gastric juices. Many chloride salts are found in nature.
Preparation of Hydrogen Chloride
Laboratory Method
In laboratory, hydrogen chloride is prepared by heating sodium chloride with concentrated sulfuric acid.
NaCl + H2SO4 → NaHSO4 + HCl
NaHSO4 + NaCl → Na2SO4 + HCl
Chemical Equipment for this Method :- Round bottom flask , thisil keep, NaCl, H2SO4, glass tube bent twice at right angles, a cork with two holes , gas jar , sprit lamp etc.
Process
Take a little sodium chloride in a round bottom flask. Tighten the flask in the stand. In this flask, a cork with two holes is put. Keep thisil in one hole and put the exhaust pipe in the other. The other end of the exhaust pipe is connected to a gas jar.
The gas is collected in the gas jar by the upward displacement of air.
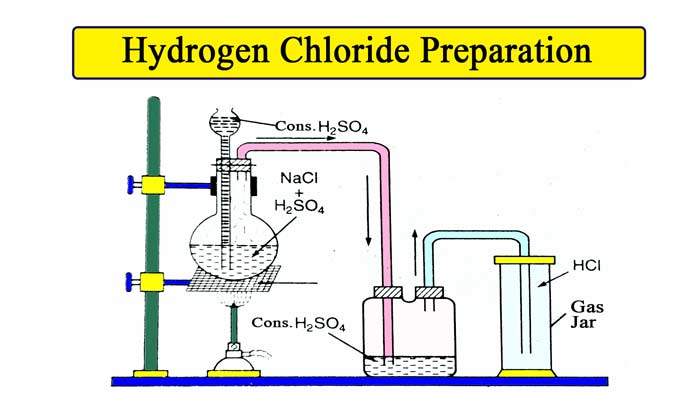
Now pour so much concentrated sulfuric acid from thisil keep that the lower violence of the keep gets immersed in the acid. The reaction takes place in a cold solution and HCl gas is formed, but after some time the rate of reaction slows down, when the flask is heated slowly, due to which the reaction starts taking place rapidly.
Drying the gas: To dry the gas, it is passed through concentrated H2SO4. To dry the gas, phosphorus pentaoxide or unslaked lime (CaO) is not used, because HCl gas reacts with both these substances.
Precautions
The lower end of thistle tube should be immersed in concentrated H2SO4.
Equipment must be airtight.
The gas jar should be used only after drying it thoroughly.
Other Method
Some other major methods of manufacturing hydrogen chloride gas are as follows –
Synthesis Method: In the presence of sunlight, hydrogen and chlorine combine to form hydrogen chloride gas.
H2 + Cl2 → 2HCl
Reaction with chlorine to water :- When chlorine gas reacts with water, hydrogen chloride gas is formed –
2H2O + 2Cl2 → 4HCl + O2
Reaction of chlorine with H2S – When chlorine reacts with hydrogen sulfide, hydrogen chloride gas is obtained –
H2S + Cl2 → 2HCl + S
Reaction of water with the chloride of aluminum, phosphorus etc.: – By reacting with aluminum or phorphosrus chloride water, HCl is formed –
AlCl3 + 3H2O → Al(OH)3 + 3HCl
PCl3 + 3H2O → H3PO3 + 3HCl
Hydrogen Chloride Test
1- It gives white smoke of ammonium chloride with ammonium dioxide.
2 – It reacts with silver nitrate to give a white precipitate of silver chloride, which is insoluble in nitric acid.
Hydrogen Chloride Properties
Physical Properties
It is a colorless and pungent gas. It is sour in taste.
This gas is highly soluble in water. 450 volumes of gas dissolve in 1 volume of water at room temperature. Its aqueous solution is acidic.
It is heavier than air and its density is 18.25.
Hydrogen chloride liquefies easily on cooling. The boiling point of liquid hydrogen is -85°C. Liquid hydrogen chloride turns into a white solid at -111.4°C.
Chemical Properties
Hydrogen chloride turns moist indigo litmus red.
This gas neither burns itself nor helps in burning.
It reacts with ammonia to produce a white smoke of ammonium chloride.
NH3 + HCl → NH4Cl
Burning sodium reacts with this gas to form sodium chloride and hydrogen gas.
2Na + 2HCl → 2NaCl + H2
It decomposes into chlorine and hydrogen by electric spark –
2HCl → H2 + Cl2
Metals like Zn, Mg, Fe etc. react with aqueous solution of hydrogen chloride gas to liberate hydrogen gas. The aqueous solution of this gas is called hydrochloric acid.
Zn + 2HCl → ZnCl2 + H2
Mg + 2HCl → MgCl2 + H2
By reacting hydrochloric acid with NaOH, Ca (OH)2 etc., salt and acid are formed –
NaOH + HCl → NaCl + H2O
Ca(OH)2 + 2HCl → CaCl2 + 2H2O
The reaction of hydrochloric acid on oxides of metals produces chloride and water of metals.
ZnO + 2HCl → ZnCl2 + H
MgO + 2HCl → MgCl2 + H2O
Hydrochloric acid reacts with a solution of silver nitrate to form silver chloride.
AgNO3 + HCl → AgCl + HNO3
HCl acid reacts with metal carbonates to form metal chloride, water and carbon dioxide.
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Hydrochloric acid oxidizes with oxidizing substances to form chlorine gas –
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
PbO2 + 4HCl → PbCl2 + 2H2O + Cl2
2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2
K2Cr2O7 + 14HCl → 2KCl + 2CrCl3 + 7H2O + 3Cl2
HCl acid reacts with lead acetate to form lead chloride and acetic acid.
Pb(CH3COO)2 + 2HCl → PbCl2 + 2CH3COOH
When concentrated HCl and concentrated HNO3 are mixed in the ratio 3 : 1 aquaregia is formed which dissolves metals like gold, platinum etc. and converts them into their chloride solution.
3HCl + HNO3 → NOCl + 2Cl + 2H2O
It reacts with barium sulfide to form barium chloride and H2S.
BaS + 2HCl → BaCl2 + H2S
Hydrogen Chloride Uses
It is used as an important reagent in the laboratory.
It is also used to make chlorine gas.
Hydrochloric acid is made from hydrogen chloride.
It is used in making aquaregia.
It is also used in making medicines.
in cleaning leather.
