How to make Hydrogen peroxide? Properties and Uses
- Barium peroxide: BaO2
- Hydrochloric acid: HCl
- Hydrated barium peroxide: BaO2.8H2O
- Barium sulfate: BaSO4
- Barium phosphate: Ba3(PO4)2
- Barium carbonate: BaCO3
- Sodium peroxide: Na2O2
- Per di-sulphuric Acid: H2S2O8
- Ammonium persulfate: (NH4)2S2O8
Thenard first created Hydrogen Peroxide H2O2 in 1818 by the action of dilute Hydrochloric on Barium peroxide.
Hydrogen Peroxide(H2O2)is found in a very small amount of rainwater and the atmosphere.
Structure of Hydrogen Peroxide:
Thenard found that if 17 parts of H2O2 are decomposed, by weight, 8 parts of oxygen and 9 parts of water are obtained. Based on this, its proportional formula is HO. Its vapor density is 17 so its weight is 34. Thus its formula is H2O2.
According to Kingjet, it is an oxidized product of water and its composition is the formula I.
According to Boyer, it is reduced production of oxygen and its structure is formula II.

Equilibrium Mixture view:
H2O2 is a tautomeric mixture of the following two formulas based on evidence in favor of the sources of Kingjet and boyer. At normal temperature, H2O2 remains in this mixture, mainly in the form of formula II.
With the help of an x-ray, it has been found that its four atoms do not exist in one place. And its structure is like an open book.
According to Abrahams, the angle between H – O bond and O – O bond is 101.9° and the length of O – O bond and O – H bond is 1.49A° and 0.97A° respectively. There is an angle of 90.2° between the hydrogen atoms.
Preparation Method:
The following are the methods of making H2O2.
Sodium peroxide:
In the laboratory, it is made by the action of dilute H2SO4 on sodium peroxide.
In a flask, sodium peroxide is slowly added to the solution of dilute H2SO4.H2O2 is formed by the reaction. Who lives in the solution. Crystals of sodium sulfate(Na2SO4.10H2O) are obtained upon cooling the obtained mixture. Sodium sulfate is also called Glauber Salt.
The Glauber filters the salt and separates it. A 20% solution of H2O2 is obtained in the filtrate. 20% solutions of H2O2 are also called Perhydrol.
Na2O2 + H2SO4 → Na2SO4 + H2O2
Sodium peroxide
Hydrogen Peroxide is obtained from the action of cold water such as ice on sodium peroxide.
Na2O2 + H2O → 2NaOH + H2O2
Barium peroxide
Barium peroxide paste contains ice-cooled dilute sulfuric or phosphoric acid. The received precipitate is filtered and separated.
BaO2 + H2SO4 → BaSO4 + H2O
3BaO2 + 2H3PO4 → Ba3(PO4)2 + 3H2O2
In this method, a hard surface is formed in the flask due to the formation of insoluble Barium sulfate or Barium phosphate. In which this reaction is carried out. This causes the reaction to slow down. This is a drawback to this method.
Merck’s Method
Barium peroxide is carried in cold water and carbon dioxide gas flows into the solution. By releasing carbon dioxide, barium carbonate precipitate is obtained which is separated. H2O2 remains in the solution.
BaO2 + H2O + CO2 → BaCO3 + H2O2
Hydrated barium peroxide
H2O2 is obtained when hydrated barium peroxide reacts with dilute sulfuric acid. Hydrated barium peroxide (BaO2.8H2O) reacts readily with sulfuric acid and the reaction does not slow down due to the formation of insoluble barium sulfate in this method.
BaO2.8H2O + H2SO4 → BaSO4 + H2O2 + 8H2O
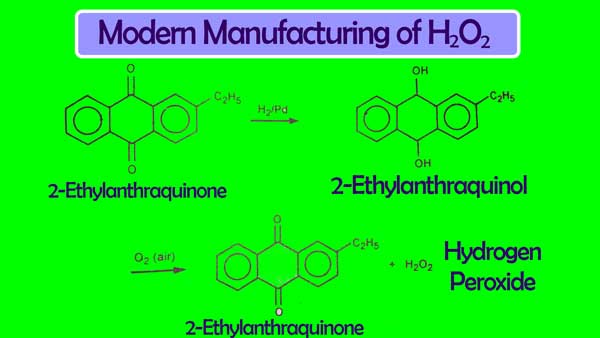
Auto-Oxidation Method
This is the modern method of making Hydrogen peroxide in large quantities. In this, 2-Ethyl-anthraquinone is dissolved in an organic solvent and it is reduced to hydrogen in the presence of Pd. The 2-Ethyl-anthraquinone derived from this action changes again to 2-Ethyl-anthraquinone. And H2O2 is obtained.
Perdisulphuric Acid(H2S2O8)
The industrial manufacture of H2O2 can be achieved by steam distillation at a low pressure of Perdisulphuric Acid. About 30% solution of H2O2 is obtained by this method.
Electrolytic Process
In this method, ammonium sulfate and dilute sulfuric acid solution are electrolytic. In electrolytic, hydrogen is obtained at the cathode and Ammonium persulfate ((NH4)2S2O8) at the anode.
(NH4)2S2O8 + H2SO4 → 2NH4HSO4
NH4HSO4 → NH4SO4– + H+
H+ + e → H Cathode
2H → H2
2NH4SO4– – 2e → (NH4)2S2O8 Anode
H2O2 is obtained by distillation of Ammonium persulfate with dilute sulfuric acid at low pressure.
(NH4)2S2O8 + H2SO4 → H2S2O8 + (NH4)2SO4
H2S2O8 +2H2O → 2H2SO4 + H2O2
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
Pure H2O2 is a colorless odorless thick and transparent fluid. In large amounts, its color appears to be light blue.
Its boiling point is 1510C and the freezing point is -0.890C. It rapidly decomposes at its boiling point. Hence its distillation is done at low pressure. Its boiling point is 680C at 26mm pressure.
Its relative density is 1.4694 at 00C and its relative density at 250C is 1.64.
It is soluble in water, ether and alcohol.
This puts blisters on the skin.
Uses of Hydrogen Peroxide
- It is used in bleaching hair, wool, etc. as bleaching.
- Being antiseptic, it is used to clean wounds, ears, teeth etc.
- It is used as propellant in rocket.
- As an oxidizer
- H2O2 is used to make hair color golden.
- It is used to prevent many substances like milk wine etc. from rotting.
- Used in retrieving the colors of old paintings of oil-dyed dyes.

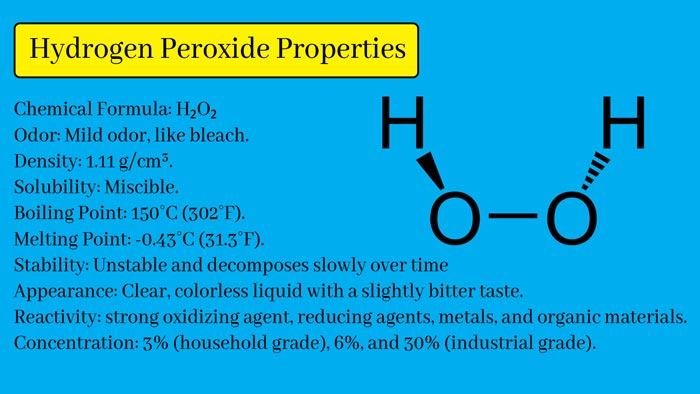
Chemical
Formula: H₂O₂
Appearance:
Hydrogen peroxide is a clear, colorless liquid with a slightly bitter
taste.
Odor:
It has a mild odor, similar to that of bleach.
Density:
The density of hydrogen peroxide is approximately 1.11 g/cm³.
Solubility:
It is miscible with water in all proportions.
Boiling
Point: 150°C (302°F).
Melting
Point: -0.43°C (31.3°F).
Stability:
Pure hydrogen peroxide is relatively unstable and decomposes slowly over time,
especially in the presence of light or certain catalysts.
Reactivity:
Hydrogen peroxide is a strong oxidizing agent and can react violently with
reducing agents, metals, and organic materials.
Concentration:
Hydrogen peroxide is commonly available in various concentrations, with the
most common being 3% (household grade), 6%, and 30% (industrial grade).
Uses:
Hydrogen peroxide has numerous applications, including as a bleaching agent,
disinfectant, antiseptic, and oxidizing agent in various industrial processes.
It is also used in the production of certain chemicals and as a component in
rocket fuel.
Safety
Precautions: Concentrated hydrogen peroxide is corrosive and can cause skin
burns and eye damage. It should be handled with care and diluted before use.
Additionally, mixing hydrogen peroxide with certain substances can produce hazardous
reactions, so compatibility should always be checked before combining with
other chemicals.