What is the ionic theory of electrolysis? Arrhenius Ionic Theory
Svante Arrhenius formulated in 1887 a theory called ionic theory that elucidated the process of electronic decompositions and other properties of electronic decompositions.
Electrolytes and Electrolysis
The substances which conduct the current stream are called Electrical conductors. Substances which do not operate the electric current are called Electrical insulators. The electrical conductors can be divided into two categories.
Metallic conductors or Electronic conductors:– In these conductors, electrons act as electrical carriers. It is clear from the study of metallic bonds that they contain loosely bound electrons or mobile electrons.
Hence all metals are electrical conductors. Similarly, graphite, some solid salts, and some oxide are also electrical conductors. There is no chemical change in the metallic or electronic conductors when they are carried out electrically.
Electrolytes:- Some substances conduct an electric current in a liquid state or in a solution. These substances have free ions in the liquid state or in the solution which act as electrical carriers. These substances are called Electrolytes.
Sodium chloride, copper sulphate and all ionic compounds soluble in water are conductors of this category and are called electrolytes. It is clear that sodium chloride(NaCl) and copper sulphate(CuSO4) are not conductors of electricity in the solid state but are conductors of electricity in fused state and aqueous solutions.
Some substances cannot be transported in liquid state or in their solution. These substances are called nonelectrolytes. Ethyl alcohol, chloroform, sugar, glucose, and urea are non-electrolytes.
These substances do not contain free ions in the liquid state or in their aqueous solutions and cannot be electrically transported from them. The number of free ions in water is so less that it is also considered non electrolytes.
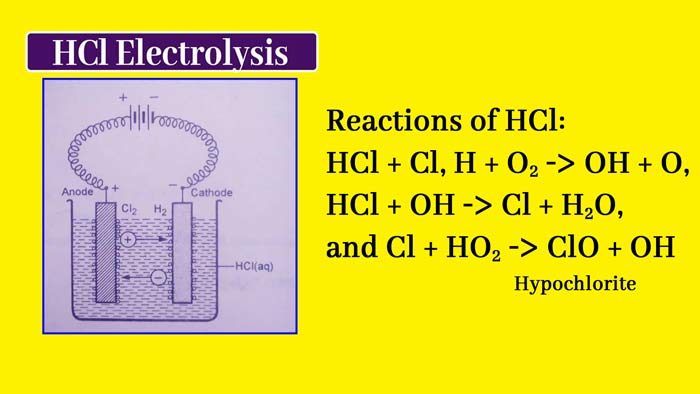
In the molten state of an electrolytes or its aqueous solution, current flows, there is a transfer of material in it and its decomposition. This reaction is called electrolysis or electrolytic decomposition.
Example: The following reaction occurs when current flows in an aqueous solution of HCl –
2HCl(aq) → H2(g) + Cl2(g)

The device used in the process of electrolytes is called electrolytic cell. In this device the liquid state of electrolytes or its aqueous solution the current is flown with the help of two metal rods. Both of these metal rods are called electrode.
The electrode connected to the positive pole of the external source is called the positive electrode and the electrode connected to the negative pole of the external source is called negative electrode. A positive electrode in an electrolytic cell is called anode and negative electrode is called cathode.
Theory of Electrolytic Dissociation
Grothus first attempted to explain the mechanism of electrolytic in 1805. After this, many scientists made many other efforts to clarify this theory.
In 1857 Clausius made his suggestions. Using these suggestions, Svante Arrhenius formulated a theory called the Theory of electrolytic dissociation or ionic theory in 1887 to clarify the process of electronic decompositions and other properties of electronic decompositions. The following is the modern form of this theory.
1) Upon dissolving a electrolytes in water or any other solvent, it breaks down into two types of electrically charged particles. This action is called ionisation or electrolytic dissociation.
Example: On dissolving sodium chloride (NaCl) in water, it gets divided into sodium ions (Na+) and chloride ions (Cl–).
NaCl ⇋ Na+ + Cl–
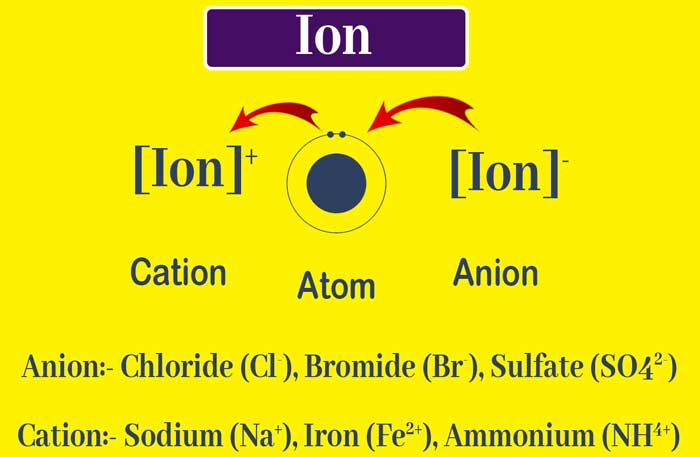
2) An atom or group of atoms with electric charge is called ion. The positively charged atom or group of atoms is called cation and the negatively charged atom or group of atoms is called anion.
3) The number of charges on an ion is equal to the valency of its atom or group of atoms.
Example: The number of charges on Na+ is 1 and the valency of Na is also 1. Thus, the number of charges on Ca++ is 2 and the valence of Ca is also 2. The charge on an ion is often called its valency.
Example: The valency of Na+ is called + 1 and the valency of Ca++ is called + 2.
4) Some number of charges of cations present in a solution of an electrolytes is equal to some number of charges of anions i.e. the solution of electrolytes is electrically neutral.
5) The action of ionization is reversible. When an electrolytes is dissolved in water, equilibrium is established between the molecules and ions of the electrolyte.
Example: If AB is an electrolytes, then by dissolving AB in water, equilibrium is established between the molecules AB and ions A+ and B–.
AB ⇋ A+ + B–
Equilibrium:- K = [A+][B–] / [AB]
Here K is a constant. Which is called dissociation constatnt or ionisation constant.
6) When an electrolytes is dissolved in water, not all its molecules are ionized. The part of the molecules of an electrolytes that dissociates as ions is called degree of ionisation or degree of dissociation.
Therefore
Amount of ionisation = number of molecules separated in ions / total number of molecules
Its value is less than 1 or 1. It is also expressed in percentage.
Percentage of Ionisation = Volume of Ionisation x 100%
7) The physical and chemical properties of an electrolytes depend on the nature and quantity of their ions.
8) The electrical conductivity of a solution of an electrolytes depends on the number of ions present in the solution and their charge. If the number of ions is high and the amount of charge is high then the electrolytes will also be high.
9) Ions behave like molecules to determine the colligative properties of a solution. Hence the effect of 1 mole of which electrolytes will be higher than the effect of 1 mole of any electrolytes on the enhancement of the boiling point, low in freezing point, decrease in vapor pressure and osmotic pressure.
If 2 ions are obtained by ionization of 1 molecule of electrolytes, the effect of 1 mole of electrolytes will be twice the effect of 1 mole of electrolytes.
Difference between Atom and Ion
The following difference between atom and ion
(1) Atoms are electrically neutral while ion is positive or negatively charged.
Example: The sodium atom has 11 electrons, 11 protone, and 12 neutron and is electrically neutral while sodium ion has 11 electrons, 11 protone, and 12 neutron and has a unit positive charge.
(2) Except for inert gases, the electronic structures of atoms of all other elements are temporary. The electronic structures of ions are permanent.

Example: Sodium atom has 1 electron in its outer shell and its electronic structure is temporary. There is 8 electron in the outer base of sodium ion and according to Octave, its electronic structure is permanent.
(3) Due to temporary electronic structure, atoms often cannot remain in free State and form molecules by reacting with other atoms.
Example:
H + H → H2
H + Cl → HCl
Due to permanent electronic structure, ion solution can remain stable and independent. They also participate in chemical reactions in the presence of suitable substances. In these reactions their electronic structure does not change or one type of permanent structure is converted into another type of permanent structure.
Example
Ag+ + Cl– → AgCl
2Cu2+ + 4I– → Cu2I2 + I2
The properties of atoms and ions are completely different due to different electronic structures.
Factors Influencing the Ionization
The amount of ionization of an electrolytes depends on the following factors –
Nature of electrolytes – Substances which dissociate almost completely in their ions in aqueous solutions are called strong electrolytes.
Strong electrolytes can be divided into three categories –
All salts like: NaCl, KNO3, NH4Cl and CH3COONa
Compounds formed by the reaction between acid and base are called salts. All salts are formed by ions. All salts dissolve almost completely in their ions in aqueous solutions and are called strong electrolytes.
Salts that are soluble in water (example: BaSO4 and AgCl) are also considered strong electrolytes. The reason for this is that whatever amount of their solubility in water gets dissociated as their ions.
Strong acid example:- HCl, HNO3 and H2SO4
Strong base example:- NaOH and KOH
Substances that have a small amount of ionization in their aqueous solutions, ie, which remain mainly in the aqueous solution in the form of applied molecules, are called weak electrolytes. Weak electrolytes can be divided into two categories-
weak acid example: CH3COOH, HCN and H2S
weak base example: NH4OH
- Electric Cell : E.M.F., Terminal Potential, Internal Resistance
- How Transistor Works : PNP and NPN Transistors
- How p-n Junction Diode works : Forward and Reverse Biasing
- Semiconductors : How Semiconductor works and Types
- X-Rays – Production, Properties, Wavelength and Uses
Nature of solvent:- The nature of solvent also affects the amount of ionization. The amount of ionization of an electrolytes varies among different solvents. The main reason for this is that dielectric constants of different solvents also differ.
The dielectric constants of a solvent are its power which reduces the attraction force in the electrically charged particles present in it. In mathematical terms it is represented as:
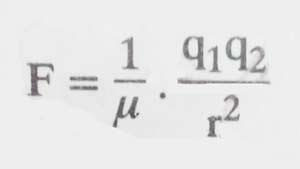
Here q1 and q2 are the quantities of charges of two oppositely charged particles, r is the distance between them, F is the force of attraction between them and μ is the dielectric constant of the solvent. It is clear that the higher the value of μ, the lower the value of F means that the amount of ionization will be higher.
Following is the value of dielectric constants of different solvents.
Water has higher dielectric constants. Therefore, in the presence of water, the amount of ionization is also high.
Concentration of a solution: The lower the concentration of a solution, the greater is the ionization of electrolyts in that solution, ie the ionization of electrolytes is inversely proportional to the concentration of the solution.
The dilute solution has a greater number of solvent molecules than the molecules of electrolytes. A greater number of solvent molecules ionizes a greater number of solute molecules. After a limit of dilution, ionization becomes stable as all the molecules that are ionized by electrolytes are ionized.
Heat: The heat increase increases ionization. The reason for this is that by increasing the temperature, the speed of the molecules increases, the attraction force between the ions decreases and the ionization increases.
Presence of even ion in the solution: If common ion is present in the solution then the amount of ionization decreases.
Example: In the presence of ammonium chloride (NH4Cl), the amount of ammonium hydroxide (NH4OH) ionization decreases. Where ammonium ion (NH4+) is common ion.
Evidence in Favour of theory of Ionisation
According to ionic theory, ions are present in the molten state of any electrolytes or in a solution. The following evidence can be presented in this favor –
X-ray diffraction studies: It is known from X-ray diffraction studies that some solids are made up of ions. NaCl does not form as a unit in a crystal of sodium chloride, but this crystal is formed in such a way that each Na+ ion is surrounded by 6 Cl– ions and each Cl– ion is surrounded by 6 Na+ ions.
The whole system has the same number of Na+ ions and Cl– ions. The fused or dissolved in the appropriate solvent reduces the attraction force between the ions, allowing them to easily move and dissociate.
Ionic Reactions: The strongest evidence for the presence of ions in an aqueous solution of electrolytes is ionic reactions.
example
A white precipitate of AgCl is obtained when HCl is added to an aqueous solution of AgNO3. This reaction is shown to be low based on ionic theory.
Ag+ (aq) + Cl-(aq) → AgCl(s)
No precipitate is obtained by adding an ethyl chloride to the AgNO3 solution because ethyl chloride does not ionize.
A green precipitate of Cr(OH)3 is obtained by adding NH4OH to an aqueous solution of CrCl3, but a green precipitate of Cr(OH)3 is not obtained when NH4OH is added to an aqueous solution of K2CrO4. The reason for this is that Cr3+ is obtained by ionization of CrCl3, whereas Cr3+ is not obtained by ionization of K2CrO4.
CrCl3 ⇋ Cr3+ + 3Cl–
K2CrO4 ⇋ 2K+ + CrO42-
Colors of Compounds and their solutions: According to ionic theory, ions are present in the solution of any electrolytes. Hence it can be assumed that the colour of the solution depends on the colour of its ions. Aqueous solution of copper sulphate is blue.
Therefore, it can be assumed that the blue colour of aqueous solution of copper sulphate (CuSO4) is due to Cu2+. If this is true, the colour of copper chloride (CuCl2) and copper nitrate [Cu(NO3)2] solution will also be blue. In fact, the solutions of copper chloride and copper nitrate are blue in colour. Therefore, the presence of Cu2+ in the solutions of these three substances is confirmed.
Similarly, the colour of cobalt ions (Co2+) is pink and the solutions of its salts are pink. Colour changes in the case of complex compounds being formed. CuSO4 is blue due to Cu2+ but K2[Cu(CN)4] is colourless.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
Colligative properties: Properties such as osmotic pressure, elevation of the boiling point and depression in freezing point are proportional to the number of solute particles in the solution.
The osmotic pressure of 0.1 M solution of sugar is the same as that of 0.1 M solution of urea. The osmotic pressure of a solution of the same concentration of sodium chloride is about two times this because one molecule of NaCl dissociates into water and gives two ions Na+ and Cl–.
NaCl ⇋ Na+ + Cl–
Heat of Neutralization: In dilute solution, any neutralized heat of the strong acid and dominant base are always the same. Its value is -13 Kcal. In fact, this is the heat of water mixed with H+ and the OH– derived from the base.
H+ + OH– ⇋ H2O + 13.7 Kcal