Isomerism: What exactly isomerism is? Types with Examples.
Isomerism Definition
“When the molecular formula of two or more compounds is similar but some or all of the properties are different, these types of compounds are called Isomer of each other and this phenomenon is called Isomerism.”
What is isomerism exactly?
Knowledge of the meanings of the terms molecular formula, structure formula, and configuration is necessary before beginning the study of isomerism. Read this post for information on molecular formula and structure formula.
How to find the IUPAC name of compounds.
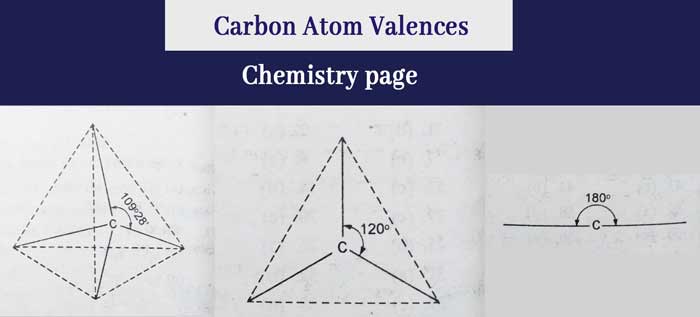
Before understanding the meaning of the word configuration, it is necessary to know the directions of the bonds made by carbon in different circumstances and the angles formed between them.
le bell and vant hoff in 1874, it was estimated that the four connectivity of carbon are directed from the centre of a rhombus to its four sides and an angle of 109°28’ between any two connectors. It has been known in this regard by experiments and the notion of orbital structure –
Isomerism: Different types of structural isomerism
1: Position isomerism 2: Ring chain isomerism 3: Metamerism 4: Functional group isomerism 5: Chain isomerism
<iframe width="834" height="469" src="https://www.youtube.com/embed/o3a7YB-_lDg" title="Isomerism: || Different types of structural isomerism || Class 11th lecture in hindi" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen></iframe>
A) If the four valences of carbon form four single bonds, these four valences of carbon are directed from the centre of a regular tetrahedron to its four vertices. And there is an angle of 109° between any two connectives. In this case the hybridization of carbon is of sp3 type.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
B) If two valences of carbon form one double bond and the remaining two valences form two single bonds, then these three bonds of carbon are in the same plane and any two bonds have an angle of 120°. In this case the hybridization of carbon is of sp2 type.
C) If the three valences of carbon form a tri-bond and the remaining valence is in the form of a single bond, then these two bonds of carbon are in a straight line and have an angle of 180° between them. The same situation occurs in the case of two bonds formed by carbon. In this case the hybridization of carbon is of sp type.
The molecular formula of a compound represents the number of atoms of various elements present in a molecule.
The structural formula of a compound describes how the various atoms present in a molecule are related to each other. In other words, the structure formula of a compound represents the different groups of atoms present in its molecule and the bonds between them.
The configuration of a compound represents the relative positions in the three dimensional region or space of the various atoms and groups of atoms present in its structure.
The structure formula of a compound gives no information about the directions of various bonds present in its molecule.
Therefore, different structures of different bonds can be displayed in the structure formula of a compound. For this reason, the structure of a compound can be represented in various forms.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
All these forms are similar to each other. In the configuration of a compound, it is necessary to display the directions of some bonds so that the relative positions of different atoms and groups of atoms in that compound can be revealed. The configuration of a compound is written in the same condition while its structure may display more than one configuration.
Example: molecule formula, structure formula and configuration of maleic acid as below:

The molecule formula of maleic acid suggests that one molecule contains four atoms of carbon, hydrogen and oxygen. The structure formula for maleic acid states that there is one carbon-carbon bond and two carboxylic group present in it. The configuration of maleic acid suggests that both the carboxylic group present in it is present on one side of the carbon-carbon bond.
Isomerism
There are many examples in carbonic chemistry when two or more compounds can be represented by the same molecular formula.
There is a difference in some or all of the properties of these compounds. Such compounds are called isomer of each other. This phenomenon is called Isomerism.
When the molecular formula of two or more compounds is similar but some or all of the properties are different, these types of compounds are called Isomer of each other and this phenomenon is called Isomerism.
Since the molecular formula of isomers are similar, the differences in their properties must be due to different arrangements of atoms present in their molecules. In other words, their structure formula or configuration must be different. Hence the definition of Isomerism can also be written as –
When the molecular formula of two or more compounds is similar but the structure formula or configuration is different, these types of compounds are called isomer of each other and this phenomenon is called isomerism.
Example: ethyl alcohol and di methyl ether are two carbonic compounds, their structure formulas are CH3–CH2–OH and CH3–O–CH3 respectively, most of their properties are different from each other but their molecular formula is similar (C2H6O).
When the molecular formulas of two or more compounds are the same and their properties differ, either their structures vary or their configurations vary. There are two types of isomerism on this basis –
Structural Isomerism
– When two or more compounds have the same molecular structure but differ in their properties due to differences in their structures, this type of isomerism is called structural isomerism and these types of compounds are called structural isomer of each other. Structural isomerism is of five types –
- Chain Isomerism
- Position Isomerism
- Functional isomerism
- Metamerism
- Tautomerism
When the molecular formulas of two or more carbonic compounds are similar but differ in the structures of chains of carbon atoms, those compounds are called chain isomers and this phenomenon is called chain isomerism.
Example 01: n-butane and iso-butane are two compounds. Each of them has a molecular formula C4H10. Their structure is as follows.

The chains of carbon atoms in these two compounds are different. Hence both these compounds are chain isomers of each other.
Example 02: n-pentane, iso-pentane and neopentane are also chain isomers. Their molecular formula is C5H12 and their structure is as follows-

Series isomerism is found in all alkanes with more than three carbon atoms. The alkanes do not have any other type of structural Isomerism.
Position Isomerism
When the molecule formulas of two or more carbonic compounds are the same, the structures of the chains of carbon atoms present in them are also similar but the positions of any atoms or groups present in the chain are different than those compounds a position isomer and this phenomenon is called position isomerism.
Example 01: n-propyl alcohol and isopropyl alcohol position isomerism. Their molecular formula is C3H8O. Their structure is as follows-

Both the above compounds have a chain of 3 carbon atoms and an alcohol group. These compounds vary in alcohol group location. N Propyl alcohol this group is located at location number 1 and iso propyl alcohol at location number 2.
Example 02: 1-butene and 2-butene status is isomerism. Their molecular formula is C4H8. Their structure is as follows-
CH3 – CH2 – CH = CH2 – 1-butene
CH3 – CH = CH – CH3 – 2-butene
Example 03: 1-chloro-propane and 2-chloro-propane have position Isomers of each other. Their molecular formula is C3H7Cl. Their structure is as follows-

Example 04: 2-pentanone and 3-pentanone have position Isomers to each other. Their molecular formula is C5H10O. Their structure is as follows-
CH3 – CO – CH2 – CH2 – CH3 2-Pentanone
CH3 – CH2 – CO – CH2 – CH3 3-Pentanone
Example 05: ortho-dichloro-benzene, meta-dicloro-benzene and para-dichloro benzene also have status Isomers. Their molecular formula is C6H4Cl2. The composition of the chain of carbon atoms is also similar, varying only in the position of a chlorine.
 Functional Isomerism
Functional Isomerism
When two or more compounds have the same molecular formula but the functional group present in them is different, those compounds are called functional isomers and this phenomenon is called functional isomerism.
Example 01: ethyl alcohol and dimethyl ether are functional isomerism of each other. Their molecular formula is C2H6O. They have alcohol and ether group respectively. Their structure is as follows.
CH3 – CH2 – OH ethyl alcohol
CH3 – O – CH3 dimethyl ether
Example 02: Acetone and propionaldehyde are functional isomers of each other.
CH3 – CO – CH3 Acetone
CH3 – CH2 – CHO Propionaldehyde
Example 03: Acetic acid and methyl formate are functional isomers of each other.
CH3 – CO – OH Acetic acid
H – CO – O – CH3 Methyl formate
Metamerism
When the molecular formulas of two or more compounds are the same, the functional group present in them is also similar but there is a difference in the number of atoms present on either side of the hetero atom present in the functional group, then these types of compounds are called metamers.
And this phenomenon is called metamerism. The hetero atom present in the functional group refers to another atom that binds on both sides, except for carbon, for example: O and N are hetero atom.
Example 01: diethyl ether and methyl n-propyl ether are metamerism of each other. Their molecular formula is similar and the functional group present in them is also similar but the number of carbon atoms of alkyl groups present on either side of the oxygen atom is different.
C2H5 – O – C2H5 diethyl ether
CH3 – O – CH2 – CH2 – CH3 methyl n-propyl ether
Example 02: Ethyl formate and methyl acetate are metamerism of each other.

Example 03: methyl n-propyl amine and diethyl amine are metamerism of each other.
CH3 – NH – CH2 – CH2 – CH3 methyl n-propyl amine
C2H5 – NH – C2H5 diethyl amine
It is clear from the above description that ether, ester and amines exhibit metamerism. Carboxylic acid, alcohol, aldehyde, ketone, nitro compound and cyanide compound do not exhibit metamerism.
Tautomerism
This phenomenon is called Tautomerism when two structural isomers mutate into each other and live in a state of dynamic equilibrium with each other. This type of isomer is called Tautomers. Actually tautomer is not two different compounds but there are two different forms of the same compound. This compound exhibits all the properties related to both these structures.
Example 01: ethyl acetoacetate participates in reactions in the following two forms.

Out of 100 molecules of ethyl acetoacetate(C6H10O3), 93 molecules contain ketone group and 7 molecules contain enol group. These two forms of ethyl acetoacetate change into each other in such a way that the above equilibrium remains.
The structures of these two forms are different. Hence ethyl acetoacetate, exhibits Tautomerism and keto form and enol form are its tautomers form.
Example 02: Nitro methane is found in the following two tautomer forms –

Example 03: Hydro cyanide acid is found in the following two tautomers forms.

The alkyl cyanide (RCN) and the alkyl isocyanides (RNC) are the isomers of each other. The existence of these isomers is evident on the basis of tautomers of hydrocynic acid.
tautomers have similar molecular skeletons. Due to the transfer of hydrogen atom to another atom in one atom of molecular skeletons, tautomers are converted into each other.
Since in this process some bonds are broken and some new bonds are formed, hence tautomerism is also called Desmotropism. Since the functional group present in tautomers is different, tautorism is a special type of functional isomerism.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
Keto – enol tautomerism: When one of the two tautomer forms is keto and the other is the enol form, this type of tautomerism is called keto-enol tautomerism.
example: The two tautomer forms of acetoacetate ester have one keto form and the other enol form and this compound exhibits keto-enol tautomerism. In order to demonstrate keto-enol tautomerism, it is necessary that the compound contains a carbonyl group (keto or aldehyde group) and at least one alpha hydrogen atom present. At least one carbon atom in the carbon atoms attached to the –CO – group. But a hydrogen atom must be present.
On this basis, acetone(CH3COCH3), Cyclohexanone(C6H10O), acetophenone (C6H5COCH3), propaldehyde(CH3CH2CHO) and Phenole(C6H5OH) can exhibit keto-enol tautomerism but benzophenone(C6H5COC6H5), benzaldehyde(C6H5CHO) does not exhibit keto enol tautomerism.
Stereo Isomerism
when two or more compounds have the same molecular formula and structure formula but differ in their properties due to differences in their configuration, this type of isomerism is called stereo isomerism and such compounds are interchangeable. Is called stereo isomer. There are two types of stereo isomerism.
- Optical Isomerism
- Geometrical Isomerism
