Isotopes, Isobars and Isotones with Examples
Isotopes, Isobars and Isotones:- It has been proved by the experiment of Mosley that the basic feature of elements is their atomic number. The chemical properties of atoms depend on their number.
If only the number of neutrons of an atom is increased then its mass will increase but there will be no change in their atomic number. Hence, there will be no change in its chemical properties as well.
Isotopes Definition
In this way, the chemical properties of all the atoms that have the same atomic number will be the same. All these atoms are called atoms of the same element.
Those atoms of the same element with different mass numbers are called Isotopes of that element.
In other words, atoms whose atomic numbers are the same but different mass numbers are called isotopes of each other.
Since the mass of an atom and its atomic weight are approximately equal. Therefore, the above definition of isotopes can also be written as this – atoms whose atomic numbers are the same but different atomic mass are called isotopes of each other.
It is clear that the number of electrons and protons in isotopes atoms are the same and the number of neutrons is different. So far 112 elements have been discovered.
The isotopes of all elements are known, except for a few new elements about which there is not enough information yet.
Isotopes Examples
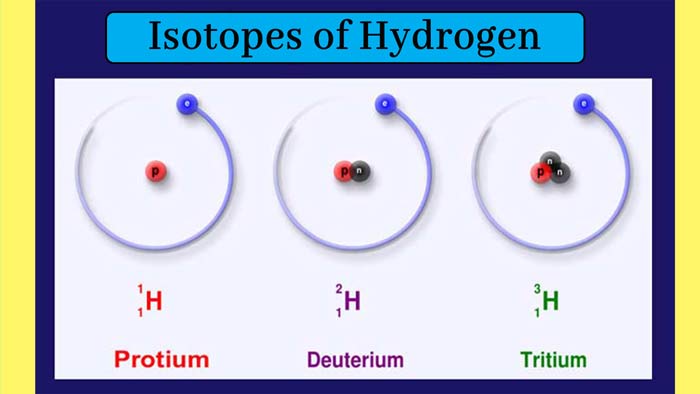
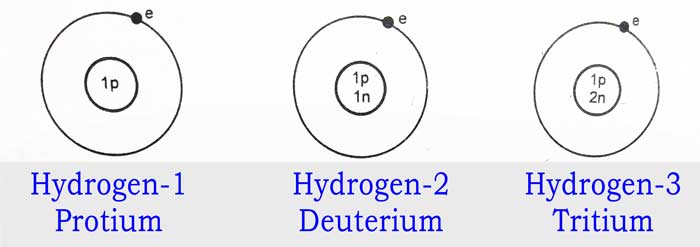
Isotopes of Hydrogen: Three isotopes of the hydrogen element are known. Their
atomic number is 1 and their mass numbers are 1, 2 and 3 respectively.
These isotopes are called hydrogen-1 or protium, hydrogen-2 or deuterium
and hydrogen-3 or tritium.
Their symbols are 1H1, 1H2, and 1H3 respectively. Each of them has 1 electron and 1
proton and the numbers of neutrons are 0, 1 and 2 respectively.
Isotopes of Oxygen: Three isotopes of the oxygen element are known. Their atomic number is 8 and their mass numbers are 16, 17 and 18 respectively. These are called oxygen-16, oxygen-17 and oxygen-18.

Feature of Isotopes
)- The atomic numbers of isotopes are the same and the atomic mass are different.
)- In isotopes the numbers of electrons and protons are the same and the numbers of neutrons are different.
)- Since the atomic numbers of isotopes are similar, the chemical properties of isotopes are similar.
Example: Light hydrogen gets water when it is burnt in air. In the same way, when heavy hydrogen is burnt in air, heavy water is obtained.
)- Physical properties of isotopes vary (density, boiling point)
)- The number of neutrons in the nuclei of isotopes varies. Hence the nuclear structure of isotopes is different. The radioactive properties of isotopes may be different due to the differing nuclear structure.
Example: carbon-12 is not radioactive while carbon-14 exhibits radioactivity.
In Mendeleff’s original periodic table, elements were placed in increasing order of atomic weight. In 1913 Mozley proved that the basic characteristics of elements are not atomic weight but atomic number and the chemical properties of elements depend on their atomic number.
Therefore, in the modern periodic table, elements are placed in the increasing function of their atomic number, therefore all the isotopes of an element are kept in one place in the periodic table, and hence they are named isotopic.
Types of isotopes
1. Radioactive or permanent isotopes – The nuclei of these isotopes are permanent and do not disintegrate automatically. The three isotopes of carbon displayed in the table, carbon-12 and carbon-13 are not radioactive.
It is called radioactivity-less or permanent isotopic. Similarly, hydrogen-1 and hydrogen-2 are one of the three isotopes of hydrogen. Hydrogen is not radioactive.
2 Radioactive or temporal isotopes – The nuclei of these isotopes are temporary. For this reason, the nuclei of the isotopes themselves disintegrate.
As a result of dissolution, radioactive rays (α, β, and γ rays) and also types of nuclei or atoms are obtained. Only the isotopic 1H3 of hydrogen and isotopic 6C14 of the carbon shown in table represent radioactive.
Uses of Isotopes
Isotopes are mainly used in the fields of scientific research, agriculture and medicine.
The most prominent examples of the use of isotopes are those in which isotopes are used as investigative elements. The use of radioactive isotopes is prominent in these examples.
Isobars
The atomic numbers of different atoms may be the same and the mass numbers may be different. Such atoms are also found whose atomic numbers are different but their mass numbers are equal.
Atoms whose mass numbers are the same and atomic numbers are different, are called Isobars to each other.
Isobars Definition
Since the mass numbers of an atom and its atomic weight are almost equal, the above definition of the logarithms can also be written as follows –
Those atoms whose atomic weights are almost the same and have different atomic numbers are called isobars to each other.
It is clear that in isobaric atoms the number of electrons, protons and neutrons are different.
All atoms with the same atomic number have the same chemical reactions and all these atoms are called atoms of a single element.
Similarly, chemical reactions of elements with different atomic numbers have different types and those atoms are called atoms of different elements.
Therefore, atoms of relatives are atoms of different elements and have different positions in the periodic table.
Isobars Examples
18Ar40 and 20Ca40 are atoms of two different elements (argon and calcium). Their atomic numbers are 18 and 20 respectively and their mass numbers are 40. Hence these atoms are isobar to each other.
6C14 and 7N14 are atoms of carbon and nitrogen respectively. They have the same mass number of 14. And the atomic number is different (6 and 7 respectively). Hence these atoms are isobar to each other.
The atomic numbers of Hydrogen – 3 (1H3) and Helium-3 (2He3) are 1 and 2 respectively and their mass number is 3. Hence these atoms are isobar to each other.
Features of Isobars
The atomic numbers of isobars are different and the mass numbers are the same.
The number of electrons, protons and neutrons in isobars varies.
The atomic weights of isobars are almost the same.
The chemical properties of isobars vary as the atomic numbers vary.
The physical properties of isobars also vary.
Along with the nuclear structure of isobars, the structure outside the nucleus is also different. Due to differing nuclear structures, their radioactive properties may be different.
Example – C-14 exhibits radioactivity while N-14 is not radioactive.
Elements in the periodic table are placed in increasing order of their atomic numbers. Therefore, the positions of isobars in the periodic table are different.
Isotones
Atoms of different elements that have the same number of neutrons are called isotones. Some of the major extracts of isotones are as follows –
Carbon-14 is nitrogen-15 oxygen-16 isotones atom. Each of them has 8 neutrons in its nucleus.
Chlorine-37 is argon-38 and calcium-40 isotones atom. Each of them has 20 neutrons in its nucleus.