Ketone Functional Group || How Ketone Bodies are Produced
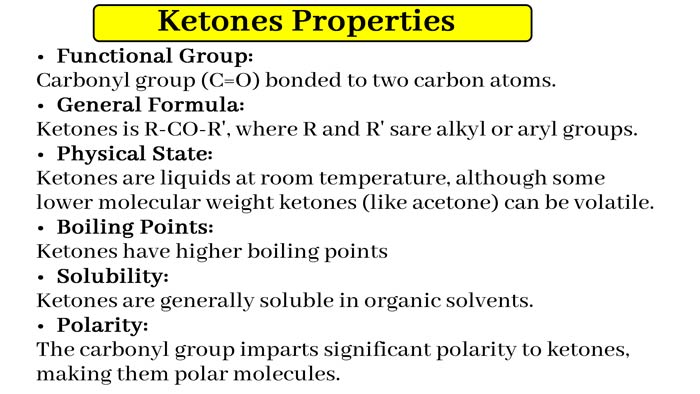
Ketone group: When −CO− group is attached to carbon atoms on both sides, it is called the Keto group or Ketonic group. And the related compound is called a Ketone.
In CH3−CO−CH3, the −CO− group is linked to two methyl groups via carbon atoms. Hence Ketones group is present in it and it is a ketone.
−CO− group presents in CH3COOH but it is connected with Oxygen of −OH group from one side. So this is not a Ketonic group.
Ketones Definition
The compounds in which the −CO− group is attached to the alkyl group on both sides are also called Alkanones. The common formula of these compounds is CnH2nO, where “n” is greater than 2 or 2. The general structure of these compounds is the formula R1−CO−R2, where R1 and R2 are alkyl groups. R and R may be the same or different, there is a difference of CH2 between two functional alkenones.
Hence all alkenones are members of the same homogeneous range. The first member of this category is acetone (CH3−CO−CH3) and the second member is methyl ethyl ketone(CH3−CO−C2H5).
Nomenclature of Ketones
Carbonyl group present in both Aldehydes and Ketones. Therefore both compounds are called Carbonyl Compounds.
In the ordinary method, Ketones are named after the name of the groups associated with the Ketonic group.
In the IUPAC method, Ketones are named by adding a “one” at the end of the corresponding alkane name. Removes e from the end of the English name and adds one. After this, the IUPAC name of that ketone is obtained after writing the appropriate and lowest number of carbon atoms belonging to the ketone group in a series of carbon atoms with the help of the rules mentioned earlier.
CH3−CO−CH3 dimethyl ketone acetone propanone
CH3−CO−CH2−CH3 methyl ethyl ketone Butanone
CH3−CO−CH2−CH2−CH3 methyl n-propyl ketone 2-Pentanone
CH3−CO−CH(CH3)2 methyl isopropyl ketone 3-Methyl-2-butanone
CH3−CH2−CO−CH2−CH3 diethyl ketone 3-Pentanone
There are some Kitones names are given below with IUPAC name:
Isomerism in Ketones:
Aldehydes and ketones with similar molecular properties exhibit interactive isomerism.
Example: The molecule C3H6O exhibits the following two functional isotopes.
C2H5−CHO(propanal) CH3−CO−CH3(propanone)
ketones exhibit location isomerism.
2-Pentanone CH3−CO−CH2−CH2−CH3
3-Pentanone CH3−CH2−CO−CH2−CH3
The ketones exhibit chain isomerism.
methyl n-propyl ketone CH3−CO−CH2−CH2−CH3
methyl isopropyl ketone CH3−CO−CH(CH3)2
Preparation of Ketones:
Oxidation of alcohols:
In the first term of oxidation of secondary alcohols, ketones are obtained.

Dehydration of Alcohol
Ketones are obtained by transferring secondary alcohol vapors over 3000C heated copper.

Fatty acids
Calcium salts of fatty acids can be made by the action of fatty acids and calcium hydroxide.
2CH3COOH + Ca(OH)2 → (CH3COO)2Ca + H2O
The ketone can be made by dry distillation of calcium salts of fatty acids.
(CH3COO)2Ca → CH3COCH3 + CaCO3
Bypassing the vapor of fatty acids to heated Magnus oxide at 300°C
Ketones are obtained from a mixture of other fatty acids except for formic acid.
R1 – COOH + R2 – COOH + MnO/3000C → R1 – CO – R2 + CO2 + H2O
CH3COOH + CH3COOH + MnO/3000C → CH3 – CO – CH3 + CO2 + H2O
The ketone is obtained as a result of the reaction of Grignard reagent and acid halides or amides.
CH3COCl + CH3MgCl → CH3COCH3 + MgCl2
CH3CONH2 + CH3MgCl → CH3COCH3 + Mg(NH2)Cl2
Gem Dihalides:
When both halogen atoms of Gem Dihalides are located on any other carbon atom other than the end carbon atom, ketone is obtained from their water decomposition.
CH3-CHCl2-CH3 + NaOH → CH3 – CH(OH)2 –CH3 → CH3COCH3
Ozonolysis of alkenes
As a result of the additive reaction of alkanes and ozone, ozonides are obtained. Ketone is obtained by water decomposition of ozonide.

Propyne to Ketones
Acetone is formed when the Propyne gas is heated and diluted in dilute sulfuric acid in the presence of catalytic mercuric sulfate.
CH3 – C ≡ CH + H2O → CH3 – CO – CH3
Properties of Ketones
Physical Properties
The water solubility of ketones decreases with the increase of molecular weight.
Ketones are lighter than water.
The boiling point of ketones increases with an increase in molecular weight.
Fluid ketones have a sweet smell.
Chemical Properties
The compounds of ketones contain a carbonyl group. The carbonyl group has a more polar character. For this reason, the carbon atom of the carbonyl group is positively charged.
Most reactions in ketones occur due to the invasion of the nucleic lubricating reagent on the carbon atom of the carbonyl group. Therefore, the relative reactivity of Ketones mainly depends on the positive charge of the carbon atom of the carbonyl group. If the amount of charge is high then the reactivity is high and if the amount of charge is low then the reactivity is low.
Due to the + I effect of the methyl group, it is the group providing the electron. Hence it reduces the positive charge of the carbon atom of the carbonyl group.
Some Ketones Reactions:
Chloroform reaction – In the presence of KOH, chloroform reacts with the ketones to form chloro-hydroxy compounds.

Ketones react with nitrous acid to form oximano ketones.
Ketone reacts with sodium or ethamide solution of sodamid to produce Sodium Salt.