Lead Definition || Lead pollution and Properties
· Galena: PbS
· Cerussite: PbCO3
· Anangleste: PbSO4
· Wulfenite: PbMnO4
· Stolzine: PbWO4
Chemical Formula
·
Zinc
Blende – ZnS
· Lead Fluorosilicate – PbSiF6
· Hexa fluorosilicic Acid – H2SiF6
· Sodium Plumbite – Na2PbO2
· Red Lead – Pb3O4
· Lead Monoxide – PbO
· Lead Hydroxide – Pb(OH)2
lead Occurrence
Lead element is found in
abundance in nature. It is the final and permanent product of the dissolution
of many natural radioactive elements. Therefore, it is also found in a small
amount of Free State.
In combined state it is found
with copper, antimony and copper pirite ores. Gelena is the main ore of lead.
Its primary component is PbS. It contains some amount of zinc
blende (ZnS) and 0.001 to 0.01% silver are also present.
Led is found in abundance in usa,
mexico, canada, australia and uk as galena. Lead in India is mainly found
in Jawar in Rajasthan and Hazaribagh
in Bihar.
Metallurgy of Lead element
Gelena is the main ore of
lead. In order to obtain lead from the galena, first of all obtain large pieces
of ore by crusher by hammering it with the hammer and by using a stamp mill.
After this the following steps are used in sequence.
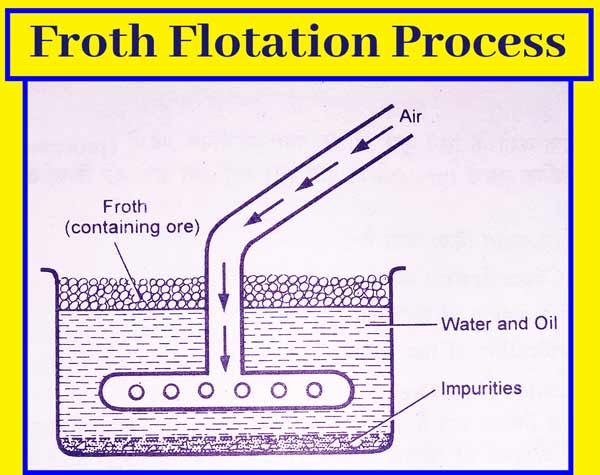
Concentration of the Ore
After grinding the galena ore,
it is concentrated by gravity separation method followed by froth floatation
method.
The fine powder of ore is poured
into a pond filled with water and pine oil to concentrate the ore by the froth
floatation method. And a strong stream of air flows in it. The concentrated ore
is obtained as froths on the surface of the fluid.
Roasting of the concentrated Ore
Concentrated ore of ganella is heated for about 6 hours in the presence of controlled heat and controlled air by placing it in a place of charge in a Reverberatory furnace. In this process, impurities of sulfur, arsenic and Antimony present in the ore are oxidized to form their oxides. Which dissociates as vapor.
S + O2 → SO2
4As + 3O2 → 2As2O3
Part of the galena is converted into lead sulfide and lead mono-oxide according to the following reactions.
PbS + 2O2 → PbSO4
PbS + 3O2 → 2PbO → 2SO2

Smelting of the Roasted Ore
For smelting the fermented ore, the fermented ore is kept in the stove of the same smelting furnace in which the ore has been fed. Stop the flow of air and make the heat bigger. On doing this the following reaction takes place
PbS + PbSO4 → 2Pb + 2SO2
PbS + 2PbO → 3Pb + SO2
The lead thus obtained is in liquid form due to the high temperature of the furnace, which is separated from the remaining ore. After re-smelting by mixing the appropriate quantity of concentrated galena ore in the remaining ore, almost the entire ore is converted into lead as per the above reactions.
If the ratio of PbS in the ore is low and impurities of SiO2 etc. are high, then some of the lead in the above action is wasted as metal feces (PbSO3).
PbO + SiO2 → PbSiO2
In this case, mix coke and chuna stone or bud in the fermented ore and heat it at high temperature in Blast furnace. The following reactions take place and almost the entire amount of lead present in the ore is obtained in liquid form in the Free State.
CaCO3 → CaO + CO2
PbSiO3 + CaO → PbO + CaSiO3
PbO + C → Pb + CO
PbO + CO → Pb + CO2
Purification of Lead
The lead obtained from the above method contains arsenic antimony tin copper bismuth and silver impurities. The following terms are used in this sequence to remove these impurities.
Oxidation: They place the impure lead in a deep ignition furnace stove and heat it for a few hours in the presence of air flow. The solid placed in the stove is converted into a liquid form.
After a while, this fluid is stirred with a rod. Most impurities are oxidized and separated into a solid layer, ie scum, over the vapor or liquid. In this activity, some lead is also oxidized to PbO. Which goes in vain as scum. The rest of the lead is mainly silver impurity.
Argenti ferrous Lead: There are two main methods of desilverisation of lead from argenti ferrous lead.
Parke’s Method: This method is based on the following principles.
- Molten zinc and lead do not dissolve. Lead is heavy in both of these, which sits down and due to the light of zinc, it floats by coming up.
- Silver dissolves more in molten zinc than molten lead.
- Egyptian metals of silver and zinc solidify earlier than molten lead upon cooling.
In this method, argenti ferrous lead is melted and melted zinc is added to it. By doing this, zinc starts to float upwards and silver dissolves in it due to its solubility in zinc.
The melted lead settles down. On cooling, zinc and silver alloy are obtained in solid form on the upper surface. Take it apart with the help of pierced spoons.
The remaining lead is added to the melted zinc again, and after cooling it repeatedly obtains a zinc and silver alloy. After repeating this action again and again, the amount of silver in the remaining lead is almost negligible.
Thus silver is separated from lead. Pure silver can also be obtained from distillation of zinc and silver alloys.
Pattinson’s Method: This method is based on the following principles.
- After melting silver and lead Egyptian metal, the lead separates into solid by cooling it.
- Repeating the above action repeatedly increases the concentration of silver in the mixture of silver and lead.
- When the silver concentration in the mixture of silver and lead becomes 2.5%, then after mixing the mixture by melting and cooling, all the mixture becomes solid together.
In this method, argenti ferrous lead is first melted. And then cool. Some of the lead is separated from the mixture of silver and lead by becoming solid. Separate it with pierced spoons. This type of action is repeated until then.
As long as the percentage is about 2.5. Initially, the amount of lead in silver was 0.001 to 0.01%. Most of the lead taken from this method is obtained in pure form and the percentage of silver in the remaining part increases.
From this remaining part pure silver can be obtained by cupellation process. If initially 100 grams of lead is taken, in which the percentage of peace is 0.1. So 96 grams of lead is obtained in pure form by this method. And a mixture of 4 grams of lead and 1 gram of silver is obtained.

Electrolytic refining: The amount of impurities in lead obtained after the above terms is much less. Remaining impurities can also be removed by electrolytic method.
In this method impure lead is used as anode, pure lead to cathode and lead fluorosilicate (PbSiF6), hexafluorosilicic acid (H2SiF6) and gelatin mixture as legitimate decomposition.
Oxidation at the anode and reduction at the cathode occurs when the current flows through the cell. Impure metal dissolves at the anode. The impurities are obtained in the form of anode mud. And pure metal begins to accumulate at the cathode.
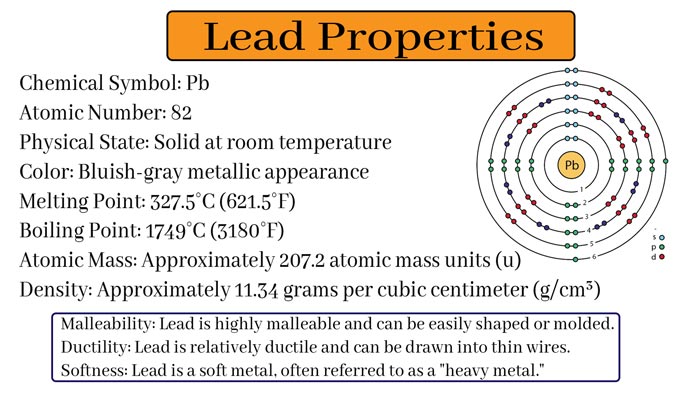
Properties of Lead
Physical Properties
Common lead is a brownish-blue metal but in pure state it has a silver-like shine.
Its melting point is 327°C and boiling point 1620°C.
Lead is a heavy metal. Its relative density is 11.34.
Its hardness is lower than most other metals, tensile is also lower but malleability is higher. This leaves black marks on the paper.
Chemical Properties
Reaction with air: Lead mono oxide or lithrage is formed when the lead is heated to high temperature in the presence of air. Which eventually transforms into red lead(Pb3O4).
6Pb + 3O2 → 6PbO
6PbO2 + O2 → 2Pb3O4
Reaction with Water: In the presence of air, this lead hydroxide is formed by reacting with water.
2Pb + O2 + 2H2O → 2Pb(OH)2
Lead hydroxide is a merger in water. Lead is a toxic element. Due to the reaction with lead water and the water solubility of lead, lead should not be used to make pipes carrying drinking water.
Reaction with Acids: Lead does not react with dilute hydrochloric acid. It reacts with concentrated hydrochloric acid to form Dihydrogen tetrachloropalladate. And releases the hydrogen gas.
Pb + 4HCl → H2[PbCl4] + H2
Lead is reacted with dilute nitric acid in this way.
3Pb + 8HNO3 → 3Pb(NO3)2 + 2NO + 4H2O
The reaction of lead with concentrated nitric acid is as follows.
Pb + 4HNO3 → Pb(NO3)2 + 2NO2 + 2H2O
Lead does not react with dilute sulfuric acid. It gives the following reaction when heated with concentrated sulfuric acid.
Pb + 2H2SO4 → PbSO4 + 2H2O + SO2
This reaction stops soon due to the formation of an insoluble PbSO4 layer. Therefore, lead and concentrated sulfuric acid do not react at normal temperature. And even at high temperatures, the reaction limit is almost negligible. For this reason, in the glass chamber method of manufacturing sulfuric acid, sulfuric acid is stored in a lead chamber.
Reaction with alkalis: It reacts with aqueous solution of sodium hydroxide to form sodium plumbite salt and hydrogen gas.
Pb + 2H2SO4 → PbSO4 + 2H2O + SO2
The reaction of lead with KOH is also similar.
Reaction with Chlorine and Sulfur: The following reaction occurs on the lead heated to chlorine with chlorine and Sulfur.
Pb + S → PbS
Pb + Cl2 → PbCl2
Uses of Lead element
Lead is an inexpensive metal. It is a useful metal due to its low melting point softness, high density and reaction under normal conditions.
Its major uses are the following.
In making lead pipes, which are used for storage of corrosive liquids and for transporting them from one place to another.
Sulfuric acid used in industrial manufacture of sis chamber
To protect telephone wires inside the ground, a layer of lead is mounted on them.
Lithrage, Red lead, Safeda and other lead compounds
In making lead recharge batteries
In making lead alloys,