What is a molecule’s easy definition? || What are examples of molecules?
molecules are composed of atoms that are combined together in a certain bonding order and spatial arrangement. This relationship between bonding order and spatial arrangement is called molecular structure.
Due to the interaction between atoms in a molecule, the physical and chemical properties of a molecule depend not only on the type and number of constituent atoms but also on the structure of the molecule.
Physical Chemistry Terms
A molecule is the smallest unit in a substance that can exist independently and be relatively stable and maintain the physical and chemical properties of the substance.
Molecules are composed of atoms, and atoms combine into molecules in a certain order and arrangement through a certain force. Taking water molecules as an example, the water is continuously separated until the properties of water are not destroyed.
The smallest unit that appears at this time is a water molecule (H2O) composed of two hydrogen atoms and one oxygen atom. A water molecule can be subdivided into two hydrogen atoms and an oxygen atom by electrolysis or other methods, but its characteristics are completely different from water.
Some molecules are composed of only one atom, and they are called monoatomic molecules, such as helium and argon. Such monoatomic molecules are both atoms and molecules.
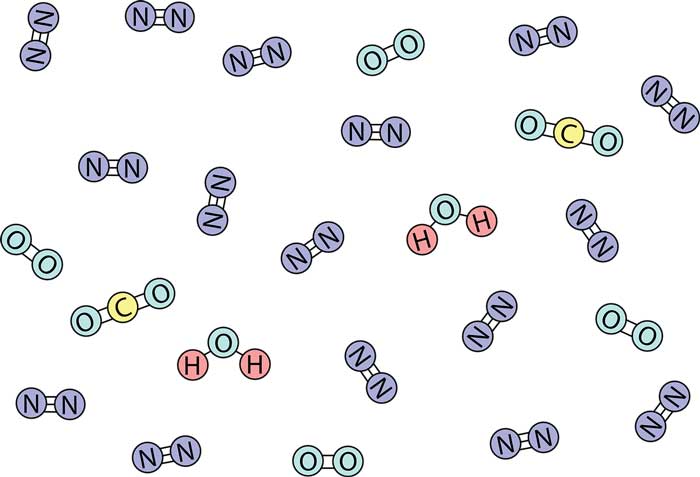
A molecule composed of two atoms is called a diatomic molecule, such as an oxygen molecule (O2) and a carbon monoxide molecule (CO): an oxygen molecule is composed of two oxygen atoms and is a homonuclear diatomic molecule; a carbon monoxide molecule is composed of an oxygen atom and one carbon atom constitutes a heteronuclear diatomic molecule.
Molecules composed of more than two atoms are collectively called polyatomic molecules. The number of atoms in a molecule can be a few, a dozen, tens, or even tens of thousands. For example, a carbon dioxide molecule (CO2) consists of one carbon atom and two oxygen atoms.
A benzene molecule contains six carbon atoms and six hydrogen atoms (C6H6), and some molecules contain hundreds of atoms, such as insulin lispro with molecular formula C257 H383 N65O77 S6.
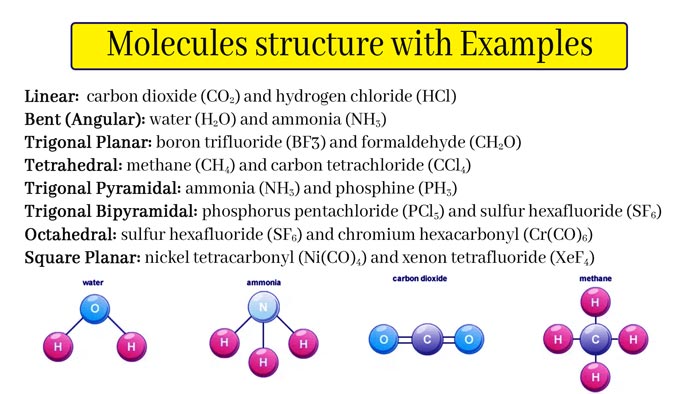
Molecules Structure
Molecular structure or molecular three-dimensional structure, molecule, molecular geometry, based on spectroscopy.
Above the data, it is used to describe the three-dimensional arrangement of atoms in the molecule. The molecular structure greatly affects the reactivity, polarity, phase shape, color, magnetism and biological activity of chemical substances.
The molecular structure is best measured at a temperature close to absolute zero because as the temperature increases, the molecular rotation also increases. Quantum mechanics and semi-experimental molecular simulation calculations can derive molecular shapes.
Solid Molecules
The structure of solid molecules can also be determined by X-ray crystallography. Larger molecules usually exist in multiple stable conformations, and the energy barrier between these conformations in the potential energy plane is higher.
Molecular structure relates to the position of atoms in space and is related to the type of chemical bonds to be bonded, including bond length, bond angle, and the dihedral angle between three adjacent bonds.
The bonding situation and spatial arrangement of atoms in molecules: the molecular structure has a decisive relationship with the physical and chemical properties of substances. The simplest molecule is a hydrogen molecule.
1 gram of hydrogen contains more than 1023 hydrogen molecules. Two hydrogen atoms in a water molecule are connected to a central oxygen atom, and the bond angle is 105.3°.
The relationship is not fixed. In addition to the translation of the molecule itself in gas and liquid, each part of the molecular structure is also in continuous motion. Therefore, the molecular structure is related to temperature. The state of the molecule (solid, liquid, gas, dissolved in solution or adsorbed on the surface) is different, and the precise size of the molecule is also different.
Molecular Structure Theory
Since there is no truly applicable molecular structure theory, the detailed structure of complex molecules cannot be predicted, but can only be measured experimentally.
Quantum mechanics believe that the orbital electrons in an atom have volatility, and mathematically processing electron standing waves (atomic orbitals) can determine the formation of bonds between atoms or groups of atoms.
The more electron orbits in an atom overlap in space, the more stable the bond formed. The quantum mechanics method is based on the combination of experimental data and approximate mathematical operations (calculated by high-speed electronic computers), which is accurate for simple systems, such as predictions on the shape of water molecules.
Another theory is to treat molecules as an electrostatic equilibrium system: the gravitational force of electrons and nuclei tends to be the largest, the repulsion between electrons tends to be the smallest, and the gravitational force of electrons in each nucleus and adjacent atoms is also very important.
In order to minimize the repulsion of the negative charge center, the system is arranged as symmetrically as possible, so when the system has 2 electron pairs, they are arranged linearly (180 °), when 3 electron pairs are arranged in a triangular plane, the bond angle 120°.
Molecules Bonding
There are three extreme types of molecular bonds, namely ionic bonds, covalent bonds, and metal bonds. The bond between the two atoms is called a localized bond. Formed by a plurality of atoms share electrons multicentre bonds referred to delocalized bonds.
In addition, there are transition-type bonds: covalent bonds whose bond electrons are biased to one side are called polar bonds, and bonds provided by one party to form bond electrons are called coordination bonds.
Through these types of bonds, atoms are combined into molecules in a certain spatial arrangement to form the molecular configuration and conformation. For example, carbon is a basic participant sharing an electron pair bond (covalent bond). The atoms of the two elements carbon and hydrogen can form hydrocarbon compounds.
CH4 in the tetrahedral structure is the simplest hydrocarbon among them, and can also form cyclic compounds, Such as cyclohexane, silicon and oxygen are the basic elements of minerals, and mica and quartz contain silicon-oxygen units. Metal atoms are sandwiched between the planes of hydrocarbon rings to form a sandwich compound.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
The basic component of protein is a difunctional molecule α-amino acid with a basic group at one end and an acid group at one end. Substances with the same chemical composition and molecular weight but different molecular structures are called isomers.
When the two isomers have the same other properties, but the optical rotation direction is opposite, this type of isomer is called an optical isomer. Available X-ray and other diffraction methods, various spectra, spectroscopy, energy spectroscopy, and mass spectrometry to determine or speculate the molecular structure.
Molecular Characteristics
1. There is a gap between the molecules. For example: take 50 ml of alcohol and 50 ml of water, and after mixing, the volume is less than 100 ml.
2. All the molecules that make up the substance are doing irregular movements without stopping. The higher the temperature, the faster the molecular diffusion, and the fastest gas diffusion in solid, liquid, and gas. Since the movement of molecules is related to temperature, this movement is called the thermal movement of molecules.
For example, clothes are easy to dry in hot weather
3. The general order of molecular diameter is 10 ^ -10m.
4. The molecule is very small but has a certain volume and mass.
5. The molecular properties of the same substance are the same, and the molecular properties of different substances are different.
Chemical formula
To reflect the true number of various atoms in a molecule, it is necessary to use chemical formulas. For example, the chemical formulas of ethylene and propylene are C2H4 and C3H6, respectively.
But the same chemical formula does not mean that two molecules are the same substance, because the arrangement and combination of atoms in the molecule, that is, the structure of the molecule, are also the factors that determine the nature of the molecule.
Molecules with the same atoms but different arrangements are called isomers. Isomers have the same chemical formula but have different characteristics due to different structural relationships. Stereoisomers are special isomers, they can have very similar physical and chemical properties, but at the same time have very different biochemical properties.
By the calculation of the laws of quantum mechanics, molecules have a fixed equilibrium geometric state-the length of the bond and the angle between them. The pure matter is composed of molecules with the same geometric structure. The chemical formula and structure of a molecule are two factors that determine its characteristics, especially its chemical activity.