How do you make Nitrobenzene? | Properties, Tests, and uses
Nitrobenzene Preparation
Nitrobenzene is obtained from the nitration of benzene. For this, benzene is heated to about 60°C with a mixture of concentrated HNO3 and concentrated H2SO4. A hydrogen atom present in the benzene nucleus is displaced by a nitro group and nitrobenzene is obtained.
C6H6 + HNO3 → C6H5NO2 + H2O
Laboratory Method
To make nitrobenzene in the laboratory, it takes approximately 50 ml of benzene in a round bottom flask. In this, a mixture of about 60 ml concentrated NHO3 and about 70 ml concentrated H2SO4 is slowly added. Stir the flask a lot.
Keep the flask temperature below 50 – 60°C. For this, keep cooling it in between. After pouring the acid mixture into the flask, place the flask on a water heater and heat it to about 60°C for about an hour.
On cooling the flask, a yellow oily layer is formed in the top of the flask mixture. Separate it with the help of a separating funnel. It is impure Nitrobenzene.
To cleanse it, first of all, wash it with sodium carbonate solution. By doing this, acidic impurities are removed. The fluid thus obtained is washed several times with water and dried by anhydrous CaCl2. The distillation of the liquid thus obtained gives pure nitrobenzene at 211°C.
Physical Properties
Nitrobenzene is a light yellow fluid. Its boiling point is 211°C. It smells like bitter almonds. It is insoluble in water and soluble in organic solvents such as alcohol, ether and benzene. Its relative density is 1.2037. It is also called oil of mirbane.
Chemical Properties
Reactions with benzene nucleus: Like other aromatic compounds due to the presence of benzene nucleus, this additive also exhibits substitution reactions.
The nitro group is a Meta director group. Hence Meta products are obtained in the substitution reactions of nitrobenzene. The nitro group is a deactivating group.
This implies that the substitution reactions of nitrobenzene are not as simple as benzene and require relatively high temperatures. Following are their major substitution reactions.
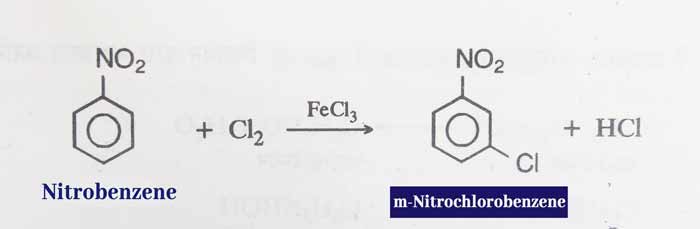
Halogenation: The reaction of nitrobenzene and chlorine in the presence of halogen carrier gives m-chloro nitrobenzene.
Nitration: Nitrobenzene gets m-dinitrobenzene when heated to about 100°C with a mixture of concentrated nitric acid and concentrated sulphuric acid.

Heating to more than 100°C temperatures produces 1,3,5-Trinitrobenzene (TNB), a strong explosive material.
Sulfonation: When heated to about 100°C with gentle sulphuric acid, it forms m-nitrobenzene sulfonic acid.

Reaction with nitro group: Due to the presence of nitro group, nitrobenzene exhibits reduction reactions. In these reactions, the nitro group is reduced and the benzene nucleus remains unaffected. The following are the major reduction reactions of nitrobenzene.
- Daily use Chemical Compounds and Their Properties
- Hard Water and Soft Water : Permutit and Anion Exchange Resins
- Properties of Water :- Purification, Sources and Uses
- Hydrogen Chloride : Preparation, Properties and Uses
- Washing Soda and Baking Soda : Difference and Properties
- Electric Cell, Terminal Potential difference, EMF and Internal Resistance
- Electrochemistry : Electrolytic cell, Mechanism and Cell Structure
- Dalton's law of partial pressure || Numeric Example
Reduction in acidic medium: Aniline is obtained when nitrobenzene is reduced by tin and HCl or Zn and HCl.
C6H5NO2 + 6H → C6H5NH2 + 2H2O
Reduction in neutral medium: Phenol hydroxyl amine is obtained when nitrobenzene is reduced by Zn and aqueous NH4Cl solution. The same product is obtained by the reaction of Al-Hg pair or Zn-Cu pair and water.
C6H5NO2 → C6H5NO → C6H5NHOH
Reduction in alkaline medium: hydrazobenzene is obtained by reduction of nitrobenzene by Zn and NaOH solutions.
C6H5NO2 + 2H → C6H5NO + H2O
C6H5NO + 2H → C6H5NHOH

Electrolytic Reduction: Upon reduction of electrolytic reduction in weak acidic medium, it forms aniline.
C6H5NO2 → C6H5NO → C6H5NHOH → C6H5NH2
Electrolytic in a strong acidic medium, it forms p-aminophenol. In this reaction, nitrobenzene reduction results in phenol hydroxylamine, which rearrange in the presence of strong acid to form p-aminophenol.

Nitrobenzene Uses
It is used in the manufacture of aniline, benzedrine, metanilic acid, TNB and other major organic compounds.
It is used as an inert solvent with high boiling point in some reactions.
In some reactions, it is used as an oxidizer.
It is also used in the name of mirbane oil as an inexpensive fragrance in soap and policy.
Nitrobenzene Tests
It is a light yellow liquid and has a bitter almond-like odor.
Aniline is obtained by heating it with tin and hydrocloric acid. Benzene diazonium chloride is obtained after mixing the obtained mixture with NaNO2 and HCl. On mixing of beta naifthole with NaOH in the obtained mixture, a bright red color is obtained due to formation of dye.