Nuclear Reactions with Examples : Nuclear Fusion and Fission with Example
The reactions in which the nucleus of an atom converts into new atoms and emits a large amount of energy are called Nuclear Reactions.
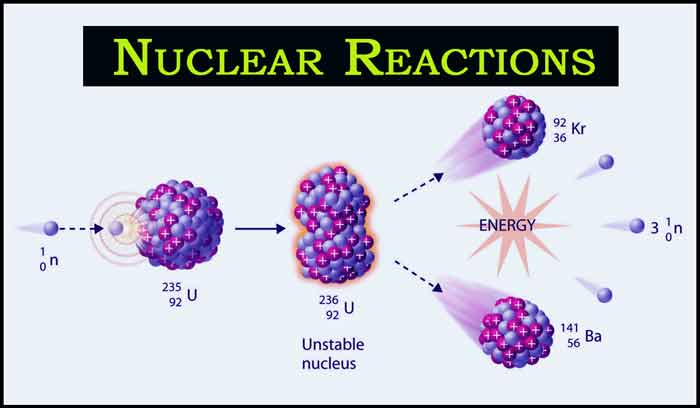
Example: When electrons are showered on Uranium-235 at a slow speed, the heavy nucleus of Uranium-235 splits into two nuclei barium-141 and krypton-92 and three neutrons are emitted. A large amount of energy is produced in this reaction.
92U235 + 0n1 → 56Ba141 + 36Kr92 + 30n1 + Large Energy
Similarly, when 92U238 is bombarded with fast neutrons, the fissile nucleus plutonium-239 is produced.

Difference between Chemical Reaction and Nuclear Reaction
Chemical Reaction:
- In these reactions, the valence electrons of the atom change but the nucleus does not change.
- The atomic number of the elements does not change in these reactions.
- In these reactions new compounds are formed and there is no difference in mass.
- The rate of a chemical reaction is affected by temperature and pressure.
- The chemical reaction can be reversible.
- Very little energy change takes place in a chemical reaction.
- In these reactions, both electrons and nuclei undergo changes.
- In these reactions the atomic number of the elements changes.
- In these completely new elements are formed and there is a decrease in mass.
- The rate of nuclear reaction is unaffected by temperature and pressure.
- Nuclear reactions are always irreversible.
- A large amount of energy is released in a nuclear reaction.
In 1939, German scientists Otto hann and F Strassman discovered that when slow-moving neutrons were fired at uranium-235, the heavy nucleus of uranium-235 splits into two nuclei of roughly equal size and with A large amount of energy is emitted.
This nuclear reaction is called nuclear fission and the energy released in nuclear fission is called nuclear energy.
Example:
The reaction of nuclear fission of uranium-235 can be represented as follows.
92U235 + 0n1 → 56Ba141 + 36Kr92 + 30n1 + Large Energy
In this fission reaction, about 84 x 107 kilojoules of energy is emitted from 1 gram of uranium-235.
Nuclear fission:
The nuclear reaction in which two very light nuclei combine to form a heavy nucleus is called nuclear fusion reaction. Fusion reactions do not occur at ordinary temperature because positively charged nuclei repel each other.
Fusion of nuclei occurs at very high temperatures. Therefore fusion reactions are also called thermonuclear reactions. At very high temperatures, lighter nuclei combine to form heavier nuclei. In which a lot of energy is generated due to the high mass loss.
Example:
When two nuclei of deutirium (heavy hydrogen) fuse together to form the nucleus of helium, a lot of energy is released in this reaction. This fusion reaction can be represented as follows.
1H2 + 1H2 → 2He4 + Energy
The formation of helium nucleus by fusion of two deutirium nuclei can be demonstrated by the following figure.

In this fusion reaction there is some loss of mass and this mass loss is converted into energy.
Example:
The fusion of 1 gram of deutirium releases 23 x 1012 joules of energy, which is much higher than the energy released when one gram of coule is burned in air. One gram of coule when burnt in air gives only 350 joules of energy.