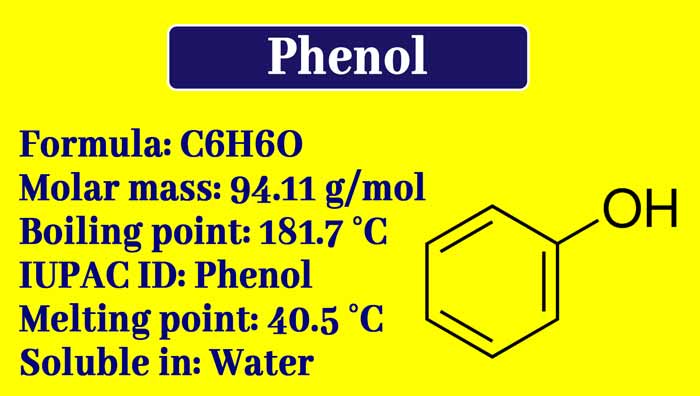
What is phenol used for? Preparation, Properties, uses, and Tests
Phenol is also called carbolic acid. The compounds in which the -OH group is directly attached to the benzene ring are called phenols or phenolic compounds and under these conditions the -OH group is called phenolic group.
Preparation
Benzene
Benzene sulfonation provides benzene sulphonic acid. Phenol is obtained by fusing sodium or potassium salt of benzene sulphonic acid with solid NaOH or KOH.

Aniline
Benzene diazonium chloride is formed when aniline is substituted at 0-5°C with sodium nitrite and hydrochloric acid. Phenol is obtained when the obtained mixture is boiled with water.
C6H5NH2 + NHO2 + HCl → C6H5 – N = N – Cl + 2H2O
C6H5 – N = N – Cl + H2O → C6H5OH + N2 + HCl
Chlorobenzene
phenol is obtained by heating chlorobenzene with aqueous NaOH at about 300°C.
C6H5Cl + NaOH → C6H5OH + NaCl
Physical Properties
The melting point of phenol is 42°C. At temperatures below 42°C, it remains solid and at higher temperatures it remains in a liquid state. Its boiling point is 182°C.
In pure state it is colorless but in the presence of light its color becomes light pink due to the small amount of oxygen being oxidized by oxygen of the air.
Its smell is pungent and distinct. It blisters when it falls on the skin. It is toxic and for this reason, is used as an antiseptic. It is less soluble in water at low temperatures but more soluble in water on heating. It is soluble in alcohol and other carbonic solvents.
Chemical Properties
Its chemical reactions can be divided into three parts.
- -OH group reactions that are similar to alcohols
- -OH group reactions that are different from alcohols
- Reactions of the benzene nucleus
-OH group reactions that are similar to alcohols
Reaction with sodium metal: It reacts with sodium metal to form sodium phenoxide.
2C6H5OH + 2Na → 2C6H5ONa + H2
Reaction with phosphorus pentachloride: It reacts with phosphorus pentachloride to form chlorobenzene.
C6H5OH + PCl5 → C6H5Cl + POCl3 + HCl
Reaction with ammonia: High pressure with ammonia in the presence of anhydrous zinc chloride and heating at 300 makes it aniline.
C6H5OH + NH3 → C6H5NH2 + H2O
Acetylation: It reacts with acetyl chloride or acetic anhydride to form acetyl derivative.
C6H5OH + CH3COCl → C6H5OCOCH3 + HCl
C6H5OH + (CH3CO)2O → C6H5OCOCH3 + CH3COOH
Alkylation: Phenol ether is obtained as a result of the action of sodium phenoxide with alkyl halide.
C6H5ONa + ICH3 → C6H5 – O – CH3 + NaI
-OH group reactions that are different from alcohols
Acidic Character: phenol is acidic and reacts with strong bases to form salts.
C6H5OH + NaOH → C6H5ONa + H2O
Phenoly compounds are acidic while alcohols are neutral. The reason for this is that the -OH group in phenol is associated with the minus-denaturing phenol group.
The oxygen atom is partially positive due to the – I effect of the phenol group and the + M effect of the –OH group. Therefore, hydrogen atom in this group can be isolated as H+.
In alcohols, the -OH group is associated with the positive charge alkyl group and does not exhibit the -OH group + M effect. Hence H+ is not obtained from alcohols.
Phenol is weak acidic. It does not react with NaHCO3 or Na2CO3. It is weaker than carboxylic acid.
Reduction by zinc powder: Benzol is obtained by heating phenol with zinc powder.
C6H5OH + Zn → C6H6 + ZnO
Oxidation: In the presence of light, oxygen is oxidized by the oxygen of air to form para benzoquinone. Even after being oxidized by acidic potassium dichromate, it forms para benzoquinone.
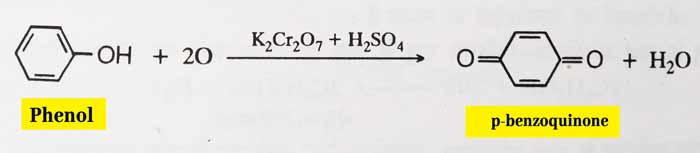
Reaction with ferric chloride: A few compounds of ferric chloride solution are added to the phenol to form a complex additive and thus give a violet color.
Reactions of the benzene nucleus
Halogenation: Halogenation of phenol occurs at a rapid rate. A white precipitate of 2,4,6-tri-bromo phenol is obtained immediately after adding bromine to phenol water.

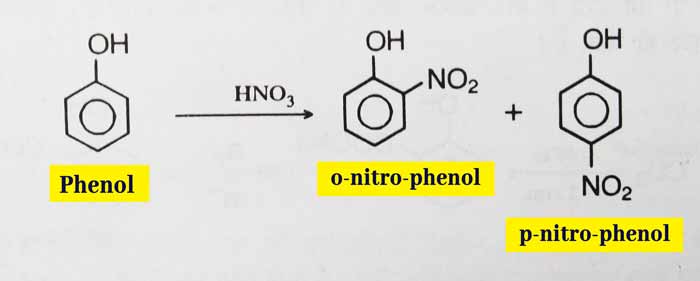
Nitration: A reaction of dilute nitric acid of phenol results in a mixture of ortho and para-nitro-phenol.
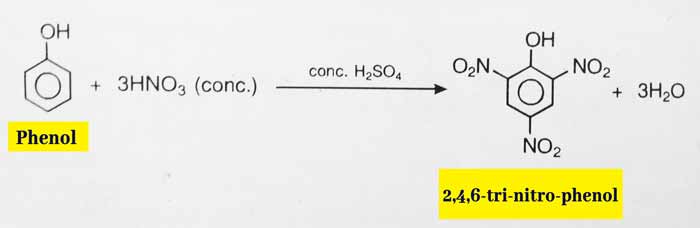
The reaction of phenol to concentrated nitric acid and concentrated sulfuric acid gives 2,4,6-tri-nitro-phenol, also called picric acid.
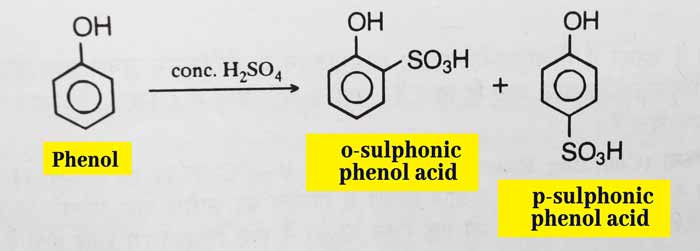
Sulphonation: A mixture of ortho and para-sulphonic-phenol acid is obtained by replicating phenol with concentrated sulfuric acid.
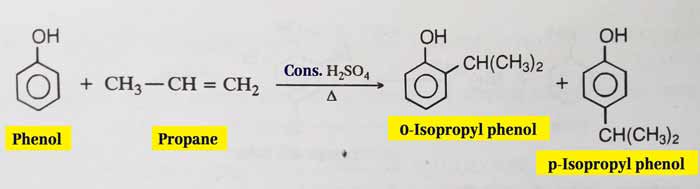
Friedel-Crafts Alkylation: It reacts with alkyl halides in the presence of anhydrous AlCl3 to form alkylation and gives a mixture of ortho and para products. Similarly, alkylation occurs when reacted with alkynes or alcohols in the presence of concentrated H2SO4.
example:
Reimer tiemann reaction: salicylic anhydride is obtained by heating phenol with chloroform and aqueous solution of KOH or NaOH.

In the above reaction, the sodium or potassium salt of salicyl anhydride is actually obtained. Salicyl anhydride is obtained after its acidic water decomposition. Salicyl acid is obtained by using CCl4 in place of CHCl3 in the above reaction.
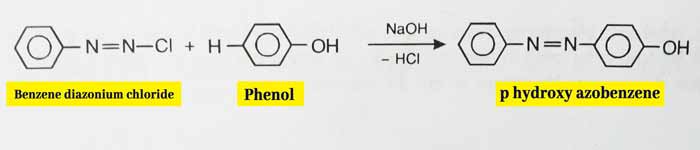
Coupling Reaction: Benzene diazonium chloride is obtained by substituting aniline with NaNO2
and HCl at 0-5°C. The coupling reaction on adding an alkaline solution
of phenol to the obtained mixture results in p hydroxy azobenzene which
is a dye and has a red color.
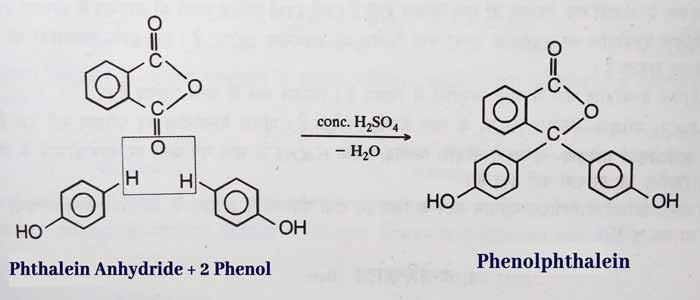
Phthalein Reaction: Phenolphthalein is formed by heating phenol with a small amount of phthalein anhydride and concentrated H2SO4.
Reaction with formaldehyde: A mixture of ortho and para hydroxy benzene alcohol is obtained by replicating phenol with formeldehide in the presence of alkali. When this reaction is carried out at high temperature, a flastic called bakelite is obtained. It is a high polymer.
Hydrogenation: Cyclohexanol is obtained by the reaction of phenol with hydrogen at high pressure and heat at about 250°C in the presence of nickel.
Phenol Uses
- The major use of phenol is in making flastic called backlite. About half of the world’s phenol consumption is used to make bakelite.
- It is used in the manufacture of salol, phenacetine, aspirin, salicylic acid, picric acid, phenolphthalein and other useful compounds. Of these, salol is used as phenacetine and aspirin drugs. Picric acid is an explosive and phenolphthalein is a useful indicator.
- Phenol is also used as an antiseptic and insecticide.
- It is also used in the manufacture of dyes
Phenol Tests
- Phenol can be identified by its distinctive odor.
- The addition of a few drops of ferric chloride solution to phenol gives a violet color.
- Phthalein test: On heating phthalein anhydride with phenol in the presence of concentrated sulfuric acid, phenolphthalein is formed which gives red color with sodium hydroxide.
- Azo dyes Tests: Benzene di azonium chloride is formed by the addition of NaNO2 and HCl to aniline. A bright red color or precipitate is obtained by adding phenol to NaOH in the obtained mixture.
- White precipitate is obtained immediately after adding bromine water to dilute aqueous solution of phenol.
- Liebermann nitroso test: green color is obtained when phenol is heated with NaNO2 and concentrated H2SO4. The red color is obtained by adding water to the obtained mixture. The green color is again obtained by adding NaOH to the obtained mixture.