Proton | Discovery, Mass, Charge | Chemistry Notes
Discovery of Proton
Goldstein discovered in 1886 that if a perforated cathode was used in the middle of the immersion tube and flowed into the gases at low pressure, a type of rays would be produced that would move from the anode to the cathode and the holes of the cathode.
It emits glow by colliding with the wall of the immersion tube. These rays are called anode rays, positive rays, canal rays or Goldstein rays.
How to learn atomic number of elements of periodic table
<iframe width="834" height="469" src="https://www.youtube.com/embed/BsblaVKcovg" title="How to learn atomic number of elements of periodic table || Short trick to learn periodic table" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" allowfullscreen></iframe>
Many scientists like W Wein, J J Thomson etc. have studied the properties of positive rays. Following are the main properties of positive rays.
- The positive rays move in straight lines.
- Positive rays are made up of microscopic particles.
- The positive rays are deflected in electric and magnetic fields.
Positive rays rotate a small and light wheel placed in its path. This proves that these rays are made up of microscopic particles and they contain kinetic energy.
The positive rays turn the electric field towards the charge plate. In the magnetic field it deflect towards the North Pole. This experiment proves that positive rays consist of positive charge particles.

The ratio of the amount of charge (e) and their mass (m) on the positive particles of positive rays derived from them by taking different gases in the immersion tube has been determined. This value (e / m) varies for different gases.
This value does not depend on the nature of the substances in the electrodes of the immersion tube.
For positive particles of positive rays of hydrogen gas, e/m is the highest value. Proton was named positive particles of positive rays derived from hydrogen by E Rutherford.
Since positive particles of positive rays from hydrogen have the highest value of e / m, these particles are lighter than positive particles obtained from other gases, so it can be assumed that positive particles from all gases contain protons.
Hence, protons are fundamental particles of all substances like electrons. Proton is fundamental particles in all substances, it is not only known by the study of the properties of positive rays but it is also proved by many other types of phenomena.
Radio activity is prominent among them. E Rutherford discovered that bombarding of alpha particles on nitrogen gas produces oxygen gases and protons are emitted.
7N14 + 2He4 → 8O17 + 1H1
Proton’s charge and mass
The value (e / m) of the charge (e) and mass (m) of the positively charged particles of hydrogen gas were determined by J. J. Thomson.
Proton charge / Proton mass = e / m = 9.58 x 104 coulomb per gram
Since the amount of negative charge on the electron is 1.603 x 10-19 coulomb, the mass of the proton can be determined if it is assumed that the amount of positive charge on the proton is also 1.603 x 10-19 coulomb.
Proton mass = e/e/m = 1.603 x 10-19 / 9.58 x 104 = 1.6726 x 10-24 gram
The mass of one atom of Hydrogen is also about 1.6726 x 10-24 gram. Hence, it is proved that the amount of positive charge of proton is equal to or 1.603 x 10-19 coulomb equal to the amount of negative charge of electron.
Since no less positive charge is found on any other particle, the charge present on the proton is also called unit positive charge. The mass of proton is 1.6726 x 10-24 grams and the mass of proton is approximately equal to the mass of one atom of hydrogen.
Origin of Anode Rays
Study of the properties of cathode and anode rays revealed that the flow of current at low pressure in gases leads to ionization of gases. Electrons and positively charged particles are formed as a result of ionization.
The positively charged particles move towards the cathode at a rapid speed and produce anode rays. In the event that the cathode is perforated, the positively charged particles come out of the cathode holes with high speed and acceleration and hit the front wall.
Discovery of Neutron
The discovery of neutron is followed by the discovery of atomic number. When the atomic number of an element is known, one gets to know the number of protons and electrons present in its atom.
The mass of an electron is 91 grams and the mass of a proton is 16 grams. On this basis, the total mass of protons and electrons present in 1 atom of an element can be determined.
The actual masses of atoms of all elements have been determined and it has been found that the actual masses of atoms of all planes are greater than the total mass of protons and electrons present in them.
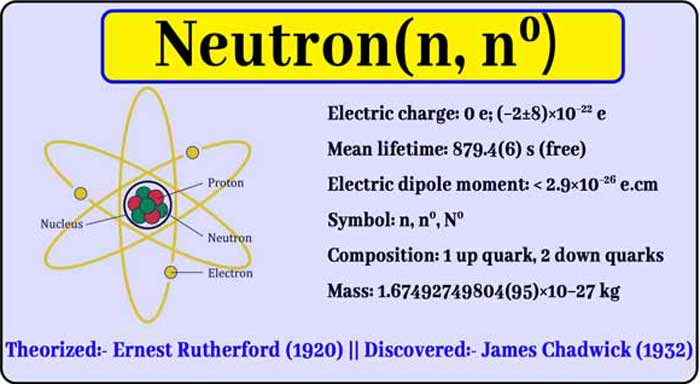
On this basis, E rutherford suggested that atoms of all elements, like electrons and protons, have a third type of particle whose weight is approximately equal to the weight of 1 atom of hydrogen and which are electrically neutral.
These particles are named neutron. Since the weight of one atom of hydrogen is approximately equal to the weight of one proton, it can also be said that the weight of 1 neutron is approximately equal to the weight of 1 proton.
Rutherford’s suggestion was first confirmed in 1932 by the English scientist J Chadwick. Chadwick found that neutron emitted when alpha particles were bombarded on some light elements such as boron, beryllium, etc.
By studying many nuclear reactions, it is now known that neutron is present in the atoms of all elements, so that neutron is also a fundamental particle of substances like electron and proton. Neutron mass has also been determined by experiments.
Rutherford has proved by scattering alpha particles that almost the entire weight of an atom is in its nucleus, so neutron in an atom is in the atom’s nucleus.
It is assumed that a neutron consists of a proton and an electron. The following action occurs when bita rays are obtained by radioactive dissolution of atoms of elements.
Sub atomic Particles
Some other particles like Positron, Meson, Neutrino, antiproton, anti-neutrino etc. are also obtained when the atom is split. These particles are temporary. Hence their importance is very less in the atomic structure.
Positron
The mass of the Positron is equal to the mass of the electron and the charge is equal to the charge of the proton. Its symbol is + 1e0.
This is achieved by the dissolution of some temporary atoms. Positron is also obtained from bombardment of photons with energies greater than 1 MeV or on other photons or particles with electric charge.
The positron is a floating particle and is charged with electrons from other substances, the total mass of the positron and electron is converted into energy according to the equation E = mc2 of eistein. The positron was discovered by Anderson.
Meson
Two types of meson particles have been discovered. Their mass is between the mass of electron and proton.
The mass of mu meson is 215 times the mass of electron and the mass of pi meson is 280 times the mass of electron.
The charge of both types of meson particles is unit positive or unit negative. Some meson particles are electrically neutral. The meson was discovered by Yukawa.
Neutrino and Anti Neutrino
Neutrino and Anti neutrino are electrically neutral and floating particles with a weight of about 0.The relative directions of their spin and linear momentum are opposite to each other and on this basis, they are called neutrino and anti neutrino. Their existence was suggested by Pauli and confirmed by Rodebeck.
Antiproton
The mass of antiproton is equal to the mass of proton and the charge of charge electron. It was discovered by Seagre.
