Sodium Hydroxide (Caustic Soda) NaOH
- Sodium carbonate – Na2CO3
- Calcium carbonate – CaCO3
- Sodium zincate – H4Na2O4Zn
- Sodium aluminate – NaAlO2
- Sodium thiosulfate – Na2S2O3
- Sodium hypophosphite – NaPO2H2
- Sodium hypochlorite – NaClO
- Sodium chlorite – NaClO2
- sodium meta aluminate – NaAlO2
This method is also called the soda-lime process. In this method sodium hydroxide and calcium carbonate are formed by heating sodium carbonate with calcium hydroxide. A white precipitate of calcium carbonate is filtered and separated.
Na2CO3 + Ca(OH)2 → CaCO3 + 2NaOH
This obtained sodium oxide has calcium carbonate inaccuracy. It is estimated by pulsing hydrochloride acid in one part of it. How much calcium carbonate is left in it. Then, by adding the appropriate amount of hot water to its aqueous solution, by heating it and then filtered, pure sodium Hydroxide is obtained in the filtered solution. It is obtained in solid form after vaporizing an aqueous solution of sodium oxide.
Electrolytic Process:
This is a modern method for the industrial manufacture of sodium hydroxide. In this method, electrolysis of an aqueous solution of sodium chloride is performed. These cells are used for the electrolytic decomposition of an aqueous solution of sodium chloride. They are given below:
Nelson Cell:
The cathode in this cell is made of steel and the anode is of graphite. An aqueous solution of sodium chloride acts as an electrolyte. This cell has a perforated groove of a U shape. Which is used as a cathode. In this cell, a graphite rod acts as an anode. In this cell, an aqueous solution of sodium chloride is filled between the anode and cathode.
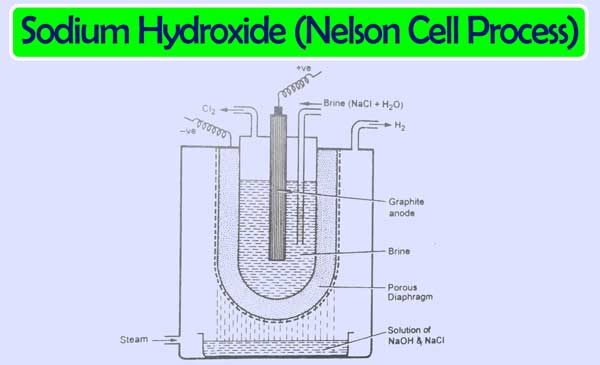
Which performs the function of electrolyte. A porous diaphragm is placed between the electrolyte and the cathode. The following actions occur if the current flows between the electrodes.
NaCl ⇌ Na++ Cl–
2H2O + 2e– → H2(g) + 2OH– Cathode
Na+ + OH– ⇌ NaOH
2Cl– → Cl2(g) + 2e– Anode
The complete and balanced equation of the reaction is as follows.
2NaCl + 2H2O → H2(g) + Cl2(g) + 2NaOH
Sodium hydroxide solution accumulates in the vessel placed under the perforated cathode. This solution contains sodium chloride as impure. Which is overcome by fractional crystallisation.
Castner Kellner cell:
Like the nelson cell, this cell is also used for the industrial manufacture of sodium hydroxide by the legal decomposition of an aqueous solution of sodium chloride.
It is shaped like a rectangular vessel. And it has three parts. A dilute aqueous solution of sodium hydroxide is filled in the middle part and a concentrated aqueous solution of sodium chloride is filled in both the outer parts.

A cathode made of iron leaves is inserted in the middle part and graphite sticks are immersed in the solution of the outer part. Which acts as an anode.
A layer of mercury is formed on the bottom of the vessel. Those who remain in contact with all the three parts of the vessel, the solutions filled in these parts are not in contact with each other. The layer of mercury can be rotated with the help of a concentric wheel so that the Na+ formed in the left and right chamber can be periodically brought into the middle chamber with the help of the mercury layer.
Sodium-ion(Na+) and chloride ion(Cl–) are present as a result of the ionization of sodium chloride in the outside portions of the vessel. When electrically flowing between electrodes, chloride ions go to the anode to form chlorine gas.
NaCl ⇌ Na++ Cl–
2Cl– → Cl2(g) + 2e– Anode
Na+ + Hg → (Na.Hg)+
2H2O + 2e → H2 + 2OH– cathode
(Na.Hg)+ → Na+ + Hg
Na+ + OH ⇌ NaOH
Hydrogen gas and hydroxide ions are obtained as a result of the reduction of H2O in the center of the vessel. In this way, there is an excess of Na+ in the outer part and there is an excess of OH– in the middle part. Sodium ions reach the surface along with the surface of mercury where these hydroxides combine with ions to form sodium hydroxide.
- How to make Hydrogen peroxide?
- What is heavy water used for?
- Isotopes of Hydrogen: Deuterium
- What is nitric acid used for?
- Do doctors still use laughing gas?
- Chemical Equilibrium: Characteristics, Types, Examples, Constant
Thus the concentration of sodium hydroxide in the middle part increases. A concentrated solution of sodium hydroxide is removed in due course. Thus this cell is used for the industrial manufacture of sodium hydroxide.
Solvay’s trough Cell:
This cell is the modified form of the Castner-Kellner Cell. In this, a six anode of graphite and about 1/3” thick surface of mercury acts as a cathode. During electrical decomposition, the surface of the mercury flows slowly from one side of the vessel to the other.

The solution of sodium chloride is kept in the cell from one side and the solution used from the other side is removed in such a way that the direction of flow of mercury and sodium chloride in the cell is the same.
Reactions:
Sodium and chloride ions are obtained as a result of the ionization of sodium chloride.
NaCl ⇌ Na+ + Cl–
Chloride ions from chlorine gases at the anode.
2Cl– – 2e– → Cl2
Sodium ions from an amalgam with mercury.
Na+ + Hg → (Na.Hg)+
(Na.Hg)+ + e– → Na.Hg
Sodium hydroxide is obtained by reacting the sodium mercury amalgam obtained from the cell with water.
Na.Hg → Na + Hg
2Na + 2H2O → 2NaOH + H2
Physical Properties
It is a white-colored solid material. Being deliquescent, it has the ability to form a solution by absorbing water vapor from the air. Therefore, after leaving open in the air, after some time, drops of fluid appear on its surface. Its melting point is 3180C and Boiling Point is 1,388°C. It is a fusion of water and alcohol. It is also called Caustic Soda. It puts painful blisters on the skin. Its aqueous solution resembles a soap solution. It is a strong base.
Chemical Properties
It absorbs air moisture and carbon dioxide when kept open in the air. By absorbing the water vapor of air, it forms a saturated aqueous solution of sodium dioxide. And by absorbing carbon dioxide, sodium carbonate is formed.
2NaOH + CO2 → Na2CO3 + H2O
When overheated, it disintegrates into its contents.
2NaOH -13000C → 2Na + H2 + O2
It is a strong base. And salt is formed by reacting with acids.
NaOH + HCl → NaCl + H2O
It also forms salts by interacting with acidic oxides.
2NaOH + CO2 → Na2CO3 + H2O
2NaOH + SO2 → Na2SO3 + H2O
It reacts with some metals to form their salts. And hydrogen gas decomposes.
Zn + 2NaOH → Na2ZnO2 + H2
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
Sn + 2NaOH + H2O → Na2SnO3 + 2H2
Cold and dilute NaOH solution makes sodium hypochlorite by reacting with chlorine.
Cl2 + 2NaOH → NaCl + NaClO + H2O
Hot and concentrated NaOH solution forms sodium chlorate by reaction with chlorine.
3Cl2 + 6NaOH → 5NaCl + NaClO3 + 3H2O
The action of Sodium dioxide with bromine and iodine is similar to that of chlorine.
When heated with sulfur, it forms sodium thiosulfate.
4S + 6NaOH → Na2S2O3 + 2Na2S + 3H2O
On heating with phosphorus, it forms sodium hypophosphite and phosphine gases.
4P + 3NaOH + 3H2O → 3NaH2PO2 + PH3
On heating with ammonium salts, it forms ammonia gas.
NH4Cl + NaOH → NaCl + NH4OH
NH4OH → NH3 + H2O
Some metals precipitate their hydroxides upon mixing with salts in an aqueous solution.
FeCl3 + 3NaOH → Fe(OH)3↓ + 3NaCl
ZnSO4 + 2NaOH → Zn(OH)2 + Na2SO4
ZnCl2 + 2NaOH → Zn(OH)2 + 2NaCl
Al2(SO4)3 + 6NaOH → 2Al(OH)3 + 3Na2SO4
The hydroxide of zinc and aluminum dissolves in excess of NaOH to form sodium ginket and sodium perminate, respectively.
Zn(OH)2 + 2NaOH → H4Na2O4Zn
Al(OH)3 + NaOH → NaAlO2 + 2H2O
Use of Sodium Hydroxide
It is used in making various inorganic and organic compounds. It is used to make alkanes from fatty acids. It is also used in the manufacture of phosphine and hydrogen.
It is used as a major reagent in the laboratory.
It is used in many industries like soap making, paper making, silk and rayon making, petroleum refining, and dyes making.
It is also used for purification of many substances after making them.