Sucrose or Sugar: The chemical name of sugar used in food is sucrose. It is also known as Cane sugar. It is mainly found in sugar cane.
Hence it is also called cane sugar. It is also found in sugar beet and some other fruit vegetables.
Manufacturing Process – Sucrose
Sugarcane is the major source of sucrose in India. Following is the term used in the industrial manufacture of sucrose or sugar from sugarcane.
Extraction of the juice
Firstly sugarcane juice is extracted using a roller mill. After this, with the help of other techniques, the maximum amount of sucrose in the residue is extracted as juice.
Purification of the Juice
There is 15 25% sucrose from the juice obtained from the above post. In this, organic and inorganic acids are present as proteins and other compound impurities. Following are the major terms used in its refinement.
Defection: By mixing 2 3% solution of lime in sugarcane juice, the ph value of the solution is reduced to about 7. The solution is heated by stream coils.
In doing so, all organic and most inorganic acids precipitate as their calcium salts, and most proteins and other compounds also dissociate as precipitates due to coagulation. Filter them and separate them.
Glucose | Fructose || Disaccharides
Structure of Diasaccharides eg. Sucrose, Maltose, Lactose Structure of Polysaccharides eg. Starch, Cellulose
Carbonation: Carbon di-oxide gas flows in the solution obtained from the above term. By doing this, the excess of lime dissociates as precipitates of calcium carbonate.
Decolourisation: The solution obtained from the above term is thick brown. When it flows over the layers of adsorbents such as animal charcoal, it becomes discolored, the colored material is absorbed by the adsorbents and the colorless fluid is obtained.
- Sulfuric Acid : Chemical Properties, Uses and Structure
- Rigid Body: Moment of Force or Torque formula
- Preparation of Sulphuric Acid by Contact process with Reaction
- Preparation of Sulfuric Acid by Lead Chamber Process
- Ozone: Tests, Uses and Ozone Structure
- What is Ozone Gas in the air? Ozone Gas Properties.
- Chemical Properties of Hydrogen Peroxide
- W
- hat is Deuterium or Heavy Water? How to make Deuterium?
Carbonation and decolourisation used in refining in India are done simultaneously. For this, sulfure dioxide flows in the solution obtained by defecation. This action is called sulphitation. In this process, impurities of acids are precipitated as their sulphate, coagulation of protien and other substances occurs and decolourisation of the solution also occurs due to the bleaching action of sulfur dioxide.
Concentration and Crystallisation: After the treatment of sugarcane juice, it is heated at low pressure. Multiple effect evaporators are used for this. In these evaporators pure juice is heated by stream coils. Separately prepared water vapor flows into the coils of the first evaporator.
The vapors from the first evaporator flow into the coils of the second evaporator, the vapors from the second evaporator into the coils of the third evaporator and likewise to the coils of the other evaporators.
Under the influence of low pressure, the action of evaporation takes place intensely. Crystals of sucrose or sugar are obtained by cooling the concentrated fluid.
Separation and drying of crystals: From the mixture of liquids and cristals obtained from the above term, the liquid crystals are separated by centrifugal machines. The cristals are dried under the influence of hot air. The remaining fluid i.e. mother liquor is called molasses. In India, alcohol is the main raw material of the industry.
Physical Properties
Sucrose or Sugar is a colorless odorless and crystal solid. Its melting point is 185°C. It tastes sweet. It is very soluble in water but very soluble in alcohol. It is optically active. And dextro is rotatory. It has a specific dextro rotatory + 66 and does not exhibit variable mutarotation.
Chemical Properties
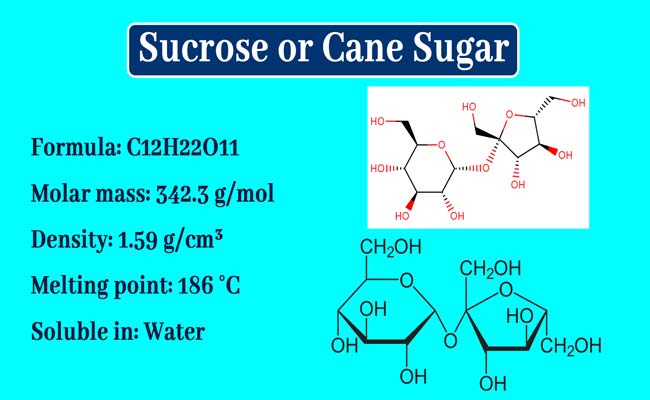
The molecule of sucrose is formula C12H22O11. Its structure formula is the following.
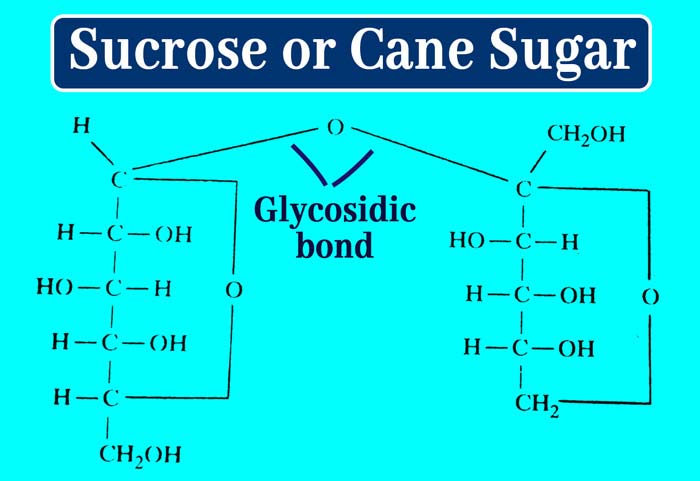
It is clear that a molecule of glucose in a molecule of sucrose is bound to a molecule of fructose between the hemiacetal group. This bond is called glycosidic bond.
Read more about Glycosidic Bong
The sucrose shows reactions of glucose and fructose in which the aldehyde or ketone group does not participate. For this reason, there is no reaction with sucrose tollens reagent, Fehling solution, hydrogen cyanide and phenylhydrazine.
The presence of glycosidic bond causes its water decomposition. Following are its major chemical reactions.
Hydrolysis: In the presence of dilute mineral acids or enzyme called invertase enzyme, sucrose water decomposes and glucose and fructose are obtained.
C12H22O11 + H2O → C6H12O6 + C6H12O6
Specific Mutarotation of sucrose, glucose and fructose is +66.5° +52.5° -92° respectively. Therefore, the aqueous solution of sucrose is a south polarity rotor and the solution obtained by its water decomposition is a left polarization rotor.
For this reason, the above reaction is called inversion of cane sugar and the mixture of sugars obtained from the above reaction is called invert sugar.
Effect of heat: On heating to 470 K temperature, sucrose molecules expel water and carbon remains as a brown material. This brown substance is used in the manufacture of sweet tablets and toffees and to give colour to the liquor industry.
C12H22O11 – 470K → 12C + 11H2O
Effect of concentrate H2SO4: Due to the sterilizing effect of concentrate H2SO4, it reacts with sucrose in this way –
C12H22O11 + H2SO4 → 12C + (11H2O + H2SO4)
In this reaction, sugar is destroyed due to the formation of carbon and its colour turns black.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
Sucrose or Sugar Uses
The most prominent use of sucrose is as a food. Carbohydrate is an essential ingredient of food. Carbohydrates are ingested mainly or in the form of sugar in food.
Sucrose or sugar is used in the preparation of many substances such as sweets, jam jelly, sweet pills, toffees, syrup used in medicines. It is also an effective food preservative. It is also used in the manufacture of substances called oxalic acid and sucrose auto acetate.