Unsaturated Hydrocarbons: Different between Alkenes and Alkynes
Those compounds are called aliphatic unsaturated hydrocarbons –
- Which are aliphatic i.e. all series of atoms present in them are open.
- Which are unsaturated, that means, at least one Carbon-Carbon two-bond or Carbon-Carbon tri-bond is present.
- In which only carbon and hydrogen elements are present.
Try it for free. No registration needed. But don’t forget to follow us our Social Pages
For more detail join us on Link to Join telegram group https://t.me/joinchat/NC9eVRt24PWWoVp… Link to Join telegram channel https://t.me/sanjaychemistrypage Registered your mail id here for the online test
<iframe width="834" height="469" src="https://www.youtube.com/embed/r__E9md1tAA" title="Crash Course NEET/JEE 2021 | Hydrocarbons | Full Chapter Revision | NCERT important concepts" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" allowfullscreen></iframe>
Those aliphatic unsaturated hydrocarbons are called alkenes with only one carbon-carbon two bond present.
Those aliphatic unsaturated hydrocarbons are called alkynes which contain only one carbon-carbon tri bond.
Alkenes
Those aliphatic unsaturated hydrocarbons are called alkenes with only one carbon-carbon two bond present.
The general formula of alkenes is CnH2n and all alkene are members of the same homogeneous range. The formula that is obtained when the value of n is 1 in the general formula CnH2n is CH2.
The four valence of carbon in this formula are not confirmed. Hence it does not represent any compound. It represents a two connective free radical named methylene radical. It is temporary.
The two methylene radicals combine to form a molecule of ethylene (C2H4). The simplest member of this category is ethylene C2H4. This compound reacts with chlorine to form an oil-like substance ethylene di-chloride.
Hence, members of this category are also called (Olfins: olefiant = oil maker). The dwo bonds present in olefins are also called olefinic bonds.
Nomenclature of Alkenes
In the IUPAC method, alkenes are named by corresponding to the end of the name of the corresponding alkene, by removing ‘an’ and adding ‘ene’.
The following are the molecule, structure formula, simple name and IUPAC name of some major alkenes.
| Molecular Formula | Structure Formula | Name | IUPAC Name |
|---|---|---|---|
| C₂H₄ | CH₂ = CH₂ | Ethylene | Ethene |
| C₃H₆ | CH₃ − CH = CH₂ | Propylene | Propene |
| C₄H₈ | CH₃ − CH₂ − CH = CH₂ | σ-Butylene | 1-Butene |
| C₄H₈ | CH₃ − CH = CH - CH₃ | β-Butylene | 2-Butene |
| C₄H₈ | CH₃ − C − CH₃ = CH₂ | iso-Butylene | 2-Methylpropene |
Isomerism in Alkenes
The first two members of the alkene class do not exhibit (ethylene and propene) isomerism. Other members of this category exhibit position isomerism, chain isomerism and geometric isomerism.
Example: The molecules formula C4H8 represents four isomer alkenes.

In these four isomerism, I and II are chain isomers, II and III are chain isomers, II and IV are chain isomers. The positions I and III are isomerism. I and IV positions are isomerism and III and IV are geometric isomerism.
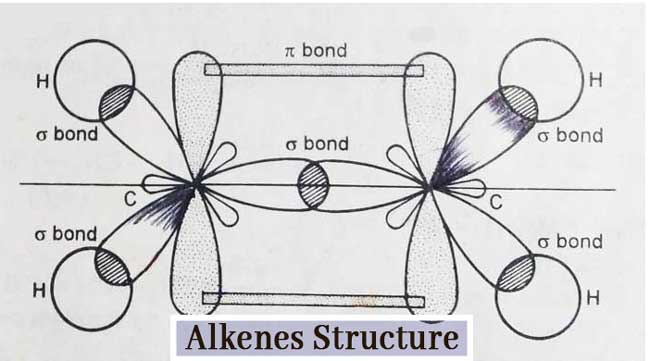
Structure of Alkenes
The structure of alkenes can be explained by the example of ethylene. Each carbon atom in ethylene (C2H4) is sp2 hybridized. Hence each carbon atom has three sp2 hybridized orbital and one pure p-orbital.
Three sp2 hybridized orbital are used to form three σ bonds. The remaining one-one p-orbital on both carbon atoms forms a π-bond by mutual lateral overlapping.

Thus the ethylene molecule consists of one carbon – carbon two bond and four carbon hydrogen single bonds. In other words, the ethylene molecule has 5 σ-bonds and 1 π-bond.
Since the π bond is formed by lateral overlap and is weaker than the σ bond made of linear overlap, the π bond is easily broken in reactions of ethylene. Other alkenes also have a 1 π bond similar to that of ethylene.
Due to the presence of π bond, these compounds are very reactive and exhibit additive reactions. This is why these compounds are called unsaturated compounds.
The sp2 hybridization has a bond angle of 120°. Therefore, the value of each H–C–H and H–C–C angle in an ethylene molecule is 120°. The ethylene molecule is planar. The alkenes have a C=C bond length of 1.34 A° and a C – H bond length of 1.09 A°. C=C bond energy is 145 Kcal per mole.
Alkynes
Those aliphatic unsaturated hydrocarbons are called alkynes, which contain only one carbon-carbon tri bond. The common formula of alkynes is CnH2n-2 and all alkynes are members of the same homogeneous range.
The formula that is obtained when the value of n is 1 in the general formula CnH2n-2 is C. It is a symbol of carbon atom.
Hence it does not represent any compound. The simplest member of this category is acetylene C2H2. The tri bonds present in alkynes are also called acetylenic bonds.
Nomenclature of alkynes
In the IUPAC method, alkynes are named by removing ‘ane’ from the end of the corresponding alkane name and adding ‘yne’. Following are the names of molecular formula, structure formula, simple formula and IUPAC of some major alkynes.
| Molecular Formula | Structure Formula | Name | IUPAC Name |
|---|---|---|---|
| C₂H₂ | CH ≡ CH | Acetylene | Ethyne |
| C₃H₄ | CH₃ − C ≡ CH | -- | Propyne |
| C₄H₆ | CH₃ − CH₂ − C ≡ CH | -- | 1-Butyne |
| C₄H₆ | CH₃ − C ≡ C − CH₃ | -- | 2-Butyne |
Isomerism in alkynes
The first two members of the alkynes category (acetylene and propen) do not exhibit isomerism.
The molecular formula C4H6 represents two position isomerism (1-butyne and 2-butyne). Other members of this category exhibit position isomerism and chain isomerism.
Example: 1-pentyne and 3-methyl 1-butyne chain is isomerism.
 Structure of alkynes
Structure of alkynes
The structure of alkynes can be explained by example of acetylene. Each carbon atom sp is hybridized to acetylene(C2H2). Hence each carbon atom has two sp hybridized orbital and two pure p-orbital. Two sp hybridized orbital are used to make two sigma-bonds and two p-orbital are used to make two π-bonds.

Thus the acetylene molecule consists of two carbon hydrogen single bonds and one carbon-carbon tri bond. In other words, the acetylene molecule has three sigma bonds and two π bonds. The π bond is weaker than the sigma bond. Like acetylene, π-bonds break easily in acetylene reactions.
Other alkynes also have two π-bonds similar to acetylene. Due to the presence of π-bonds, these compounds are highly functional and exhibit additive reactions. This is why alkynes, like alkenes, also fall into the category of unsaturated compounds.
The bond angle in sp hybrids is 180°. Therefore, the value of each H–C–C angle in an acetylene molecule is 180°. And the acetylene molecule is linear. The alkynes have a C≡C bond length of 1.20 A° and C – H bonding length of 1.09 A°. The C≡C bond energy is 192 Kcal per mole.