Valency of Elements || How to Find Valency || What is the Valency of the atom?
Valency is determined by the number of electrons an atom needs to gain, lose, or share to achieve a stable electron configuration.
For main group elements (Groups 1, 2, 13-18), valency often corresponds to the group number.
Group 1 elements (alkali metals) have a valency of +1, Group 2 elements (alkaline earth metals) have a valency of +2.
Elements in Group 17 (halogens) have a valency of -1, while those in Group 16 have a valency of -2.
Transition metals can have variable valencies due to the availability of multiple oxidation states.
Valency can be used to predict the formulae of compounds formed between elements.
Noble gases typically have a valency of 0, as they have a stable electron configuration.
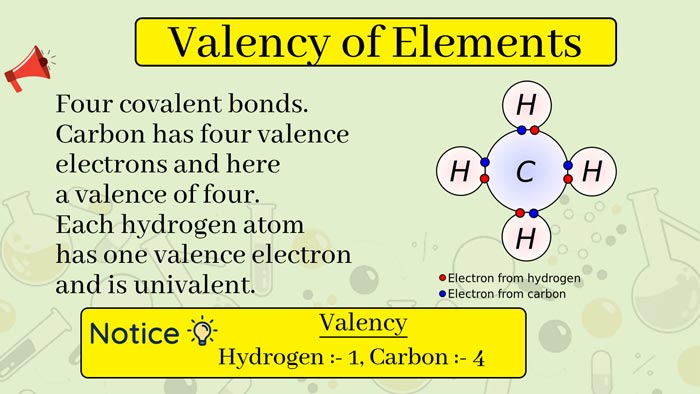
Valency of elements is the property that appears when one atom of an element combines with atoms of other elements.
In general, the valence of a valence is equal to the number of electrons that each atom gains and loses when it is combined, that is, the number of electrons that gain and lose when the element can reach a stable structure, which is often determined by the electron arrangement of the element, mainly the outermost electrons.
Valence Bond Theory
Chemical Bonding and Molecular Structure
<iframe width="1200" height="675" src="https://www.youtube.com/embed/VZ455-ypuDA" title="Chemical Bonding and Molecular Structure #15 | Valence Bond Theory | VBT | Energy Concepts" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" allowfullscreen></iframe>
It is offset by the number of electrons or shared electron pairs of atoms in the material.
Valency of elements means the number of atoms gaining and losing electrons when atoms combine with each other.
Valence is also a property that elements exhibit when forming compounds.
When elements are combined with each other, the ratio of the number of reactant atoms is not fixed but is determined according to the number of electrons in the outermost layer of the atom. For example, a sodium ion (with a valence of +1 and an electron lost) must be combined with a chloride ion (with a valence of -1 and an electron is obtained).
A magnesium ion (with a valence of +2 and two electrons lost) must be bound to two chloride ions. If the valence algebraic sum of the ions of the formed compound is not zero, the outermost electron layer of the anion and cation constituting the ionic compound and the atom of the covalent compound molecule cannot be made into a stable structure. In this way, stable compounds cannot be formed.
The concept of valence comes from this, then the number of elements outside the nuclear electrons combined with each other determines the valence of this element. The valence is set to facilitate the expression of the number of atoms that are combined with each other. When studying valence you should understand the rules for the valence of elements in compounds.
- Ammonia Formula || why ammonia is toxic || Ammonia Poisoning
- Why Ozone Layer is Important || Ozone Layer Depletion
- What is the Concentration of solution || How Concentration Affects Reaction
- Why Carbon Cycle is Important || How it Works
- Haloalkanes and Haloarenes NCERT Solutions || Haloalkane Structure
- Carbon Dioxide Cycle and Formula || How Carbon Dioxide is Produced
The predetermined elemental molecule where valency element is zero, regardless of ionic compounds or covalent compounds, n-valence anion algebra and its composition are zero. Ionic compounds, Example: NaOH (sodium n-1 is the valence of divalent, hydroxide ion valence is a negative monovalent, mutually offset by a zero-valent compound such wording on the establishment)
Valence- the ability of atoms to form chemical bonds. It is a chemical property that forms each other element in a stable compound. (That is, the ability to achieve the ability of each element to form a stable structure)
Note: The “valence” of an element is an important property of an element, which is manifested only when combined with other elements. That is when the element exists in a free state, that is, when it is not combined with other elements to form a compound, the valence of the elemental element is “0”. For example, metals such as iron, nonmetals such as carbon, and rare gases such as helium.
Valency of Elements
+1
H, Li, Na, K, Rb, Cs, Ag, Au, Hg, In, Tl, N
+2
Be, Mg, Ca, Sr, Ba, Cu, Ra, Zn, Cd, Hg, Cr, Mn, Fe, Co, Ni, C, Sn, Pb, N, S, O
+3
B, Al, Ga, In, Tl, Sc, Y, La-Lu, N, P, As, Sb, Bi, Cr, Fe, Co, Ni, Au; Ce, Ac
+4
C, Si, Ge, Sn, Pb, Ti, Zr, Hf, Ce, Th, Mn, Tb, N, S
+5
N, P, As, Sb, Bi, V, Nb, Ta
+6
S, Se, Te, Cr, Mo, W, U, Mn, Fe
+7
Cl, Br, I, Mn, Tc, Re
+8
Xe, Ru, Os
-1
F, Cl, Br, I, O
-2
O, S, Se, Te
-3
N, P, As, Sb
(Note: the valence of the atoms in the element is 0)
Special Atomic Group
Nitrate root NO 3 – : monovalent -1
Sulfite SO32- : -2
Bisulfite HSO3 – : monovalent -1
Sulfate SO42- : -2
Carbonate CO32- : -2
Perchlorate ClO3– : monovalent -1
Hydroxide OH – : monovalent -1
Ammonium NH4+ : +1 Valence
Phosphate PO43- :-3
Bicarbonate HC033– : monovalent -1
Manganate MnO42- : -2
Permanganic acid root of MnO4 – : monovalent -1
Root superoxide O2 – : monovalent -1
Peroxygen O22- : -2
Acetate CH3COO-: -1
Dihydrogen phosphate H2PO4– :- 1 valence
(Note: the formula the same, but not necessarily the same root, as manganese permanganate was +6, permanganate manganese as a divalent +7)
Fluorine, chlorine, bromine, and iodine: -1 (Acids of hydrofluoric acid, Hydrochloric acid, Hydrobromic Acid, and Hydroiodic Acid)
Non-metal Valency of elements
Since the number of electrons in the outermost layer of the metal element is mostly less than 4, it is easy to lose the outermost electron in a chemical reaction and show a positive valence, that is, the valence of the metal element is generally positive (very few metals can show negative valence (Such as antimony, -3 valences in InSb). When a non-metal element is combined with a metal element, an electron is usually obtained, and the valence is negative.
However, when several non-metal elements are combined, those with lower electronegativity will show positive valence. For example, oxygen is the second-highest electronegativity element and usually shows a -2 valence. But when it encounters the most electronegative fluorine element, it will show a valence of +2, forming OF2, and oxygen difluoride.
Calculation law
After giving the formula, if you know the valence of an element, you can multiply the valence by the number of atoms in the molecule. Because the electrical property of the valence is zero, the product of the valence of the previous valence and the number of atoms of the element is divided by the number of atoms of another element in the molecule to obtain the valence of the other element.
Given the valence of two elements, find the least common multiple of the absolute value of the valence. Then divide the least common multiple by the absolute value of the valence to find the number of atoms in the molecule.
Representation of valence: positive and negative valences should be marked directly above the element symbol with +1, +2, +3, -1, -2 … 0, etc. (Such as Na+1)
Determine the valence of the elements in the compound
(1) There are positive and negative valences
(2) The oxygen element usually shows -2 valence.
(3) The hydrogen element usually shows a +1 valence.
(4) When a metal element is combined with a non-metal element, the metal element shows a positive price and the non-metal element shows a negative price (generally, the positive price is written in the front and the negative price is written in the back).
(5) Some elements may have significantly different valences in the same substance.
(6) The algebraic sum of the positive and negative valences in the compound is 0.
(7) The valence of an element is a property that an element’s atom exhibits when forming a compound. Therefore, in elemental molecules, the valence of an element is 0.
How to find Valency of elements
Valence is a property of an element that is manifested only when the elements are fused to each other. The algebraic sum of positive and negative valences in a compound is equal to zero, which is the criterion for valence. Generally speaking, there are the following methods:
Chemical formula or radical formula
1. The formula for the Valency of elements A in AmBn compound:
(The valence of element B × the number of atoms of B) / the number of atoms of A
2. Find the valence formula of the unknown valence element in the multi-component compound:
(Algebraic sum of valences of elements of known valence) / Number of atoms of elements of unknown valence
3.Determine the Valency of elements (or an atomic group) based on the number of positive and negative charges.
In the radical formula, the algebraic sum of the total valences of positive and negative valences is equal to the number of positive and negative charges carried by the radical formula.
Exercise1: The chemical formula (molecular formula) of salt is Rm (SO4) n. What is the valence of R?
Element mass ratio
1. (Relative atomic mass of element A × valence of element B) / (relative atomic mass of element B × Valency of elements) = mass ratio of element A / mass ratio of element B
2. The mass ratio (or percentage composition) of element A × the valence of A / relative atomic mass of A = the mass ratio (or percentage composition) of element B × the valence of B / relative atomic mass of B
Exercise2: In a 7: 4 mass ratio of nitrogen and oxygen, what is the valence of nitrogen?
Mass ratio
(The valence of B × the relative atomic mass ratio of A) / (the valence of A × the relative atomic mass ratio of B) = mass ratio of element A / mass ratio of element B
Exercise3: The ratio of the relative atomic mass of elements A and B is 2: 1 In a compound consisting of only these two elements, the mass ratio of elements A and B is 2: 3, where B is negative n-valent, then A is in this compound What is the Valency of elements?
Find the valence
1. If B is -2 and C is -1, when A is odd, then A is:
(2AC type quantity-AB type quantity) / (C type quantity x B price-B type quantity x A price)
2. If B is -2 and C is -1, when A is even, then A is:
2 (AC type quantity-AB type quantity) / (C type quantity x B price-B type quantity x A price)
3. If the valences of B and C are the same, the valence of A is: (AC formula quantity-AB formula quantity) / (C formula quantity-B formula quantity)
Determine method
Chemically, the valence is used to indicate the number of interatomic compounds. It is an important property of elements. Determining the valence of an element is a basic skill that junior high school students should master. Now it is summarized in the scope of junior high school in order to help students learn.
Another Element Method
Example 1:- Determine the valence of the Mn element in the compound K2MnO4.
Analysis: Let the valence of the Mn element in the compound be + x valence, according to the principle of zero and algebraic sum of the valence of each element in the compound, there are 2 × (+1) + 1 × (+ x) + 4 × (-2) = 0 The solution is x = 6
Therefore, the valence of the Mn element in K2MnO4 is +6.
Electronic layer structure method
Example 2:- There is one electron on the outermost layer of the atom of element X, and six electrons on the outermost layer of the atom of element Y. The chemical formula of the compound that can be formed by the two elements X and Y is?
A. XY B. X2Y C. XY2 D. X3Y
Analysis: The key to this question can be said to be to first determine the valence of the two elements X and Y when forming a compound. Because the outermost layer of X has only one electron, the highest positive price is +1, and the outermost layer of Y is 6 electrons, which is 2 away from the stable structure of 8 electrons.
Therefore, the lowest negative price is -2, so X, Y The molecular formula of the formed compound is X2Y, and B should be selected.
Mass fraction method
Example 3:- The relative atomic mass of an element is 59, the mass fraction of the element in the oxide is 71%, and its valence is?
A. +1 B. +2 C. +3 D. +4
Analysis: Let the chemical formula of the oxide of this element be RxOy
59x / (59x + 16y) * 100% = 71%
Solve x / y = 2: 3
Therefore, the chemical formula is R2O3, and the R valence is +3, so choose C.
Conservation of mass
Example 4:- A metal oxide reacts with a sufficient amount of hydrochloric acid, and the ratio of the number of molecules of chloride to water is 2: 3, then the valence of the metal is?
A. + 1B. + 2C. + 3D. +4
Analysis: Suppose the chemical formula of the generated chloride is RClx, according to the ratio of the number of molecules in the title RClx: H2O = 2: 3 According to the law of conservation of mass, it can be known that the atomic type and number of each element before and after the reaction are unchanged, and H and Cl in the product are unchanged.
The ratio of the number of atoms in should also be 1: 1, so the value of x is 3, then the valence of R is +3, choose C.
Relative molecular mass
Example 5:- The relative molecular mass of the oxide of a metal element is M, and the relative molecular mass of the chloride in the same valence state is N, then the valence value of the element is?
Analysis: Let the element valence be + x, and the relative atomic mass be MR
(1) If x is an odd number, the chemical formula of the oxide is R2Ox and the chemical formula of the chloride is RClx.
2MR + 16x = M (1)
MR + 35.5x = N (2)
(2) * 2-(1) The value of x is
(2) When x is an even number, the chemical formula of the oxide is ROx / 2 and the chemical formula of the chloride is RClx.
MR + 35.5x = N (4)
In summary, A and D should be selected.
Quality relationship method
Example 6:- A metal element ag with a relative atomic mass of M reacts with a sufficient amount of dilute sulfuric acid to generate Bg hydrogen, and the valence of the metal element in the reaction is?
Analysis: Let the metal valence in the reaction be + x valence, then the metal element has the following relationship with the formation of H2:
2R ~ xH2
2M 2x
ab
Therefore, B should be selected.
Discussion of related factors
Example 7:- The outermost electron number of M atom of an element is less than 5, the chemical formula of its oxide is MxOy, and the chemical formula of chloride MClz. When y: z = 1: 2, the valence of M maybe?
A. +1 B. +2 C. +3 D. +4
Analysis: The valence of M is numerically equal to the value of z
Such as y = 1z = 2 (reasonable)
y = 2z = 4 (reasonable)
y = 3z = 6 (does not match the outermost electron number less than 5)
Therefore, B and D should be selected.