white phosphorus: Preparation, Properties, Structure, and uses
White Phosphorus
Phosphorus is a catalytic element. It is easily oxidized by the presence of oxygen in the air. Therefore, it is not found in an independent state. In nature, it is found mainly in its phosphate compounds(PO43-). Some of the main minerals in which Phosphorus is present.
- Phosphorite (Phosphate rock) Ca3(PO4)2
- Fluor-apatitie 3Ca3(PO4)2.CaF2
- Chlor-apatite 3Ca3(PO4)2.CaCl2
- Wavellite 4AlPO4.2Al(OH)3.H2O
- Vivianite Fe3(PO4)2.8H2O
Phosphorus is an essential component of all organisms. Phosphorus is a major component of bones and teeth in the form of calcium phosphate (Ca3(PO4)2).
Phosphorus Preparation:
Phosphorus is mainly made from the bone-ashes or Phosphate Rock. There are two main methods of making Phosphorus from bone-ashes or Phosphate Rock.
- Retort Process
- Electrothermal Process
Retort process is the old method and Electrothermal Process is the modern method. Both of these methods give white Phosphorus and Phosphorus.
Retort Process
In this method, heats the fine powder of bone ash or phosphorite with sulfuric acid. On doing this, orthophosphoric acid and Calcium sulfate are formed.
Ca3(PO4)2 + 3H2SO4 → 2H3PO4 + 3CaSO4↓
Continuous Calcium Sulfate is filtered and taken apart. And the filtered solution evaporates, converting orthophosphoric acid into Meta phosphoric acid. It is heated in a soil made of fireproof Retort with coke.
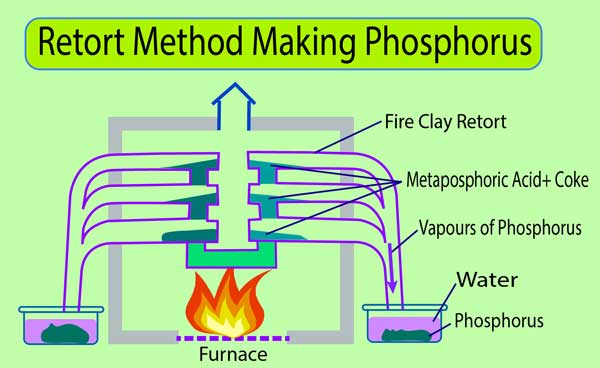
Meta phosphoric acid is reduced by coke to form phosphorus. The phosphorus evaporates and begins to exit from the retort. These vapors flow into the water and cool it, which makes the phosphorus collect as a solid on the lower surface in the water.
H3PO4 → HPO3 + H2O
4HPO3 + 10C → 4P (P4) +10CO + 2H2O
Electrothermal Process:
In this method, the fine powder of Bone-ash or phosphorus is mixed with coke and sand and heated in an electric furnace. In the electric furnace, this mixture is slowly poured from the top with the help of a rotating screw. The electric furnace generates high heat (1500-35000C) by generating an electric arc between two carbon electrodes.
Ca3(PO4)2 + 3SiO2 → 3CaSiO3 + P2O5
P2O5 + 5C → 2P + 5CO
Calcium Silicate accumulates in the form of a slag under the reactors in the electric furnace. Which is removed in due course of the exit gate.

The phosphorus made of the agent evaporates out of the furnace by the drainage. The phosphorus is cooled by caring for it in water. In this way, phosphorus gets deposited at the bottom of the pot filled with water.
Purification of Phosphorus:
The phosphorus obtained from the above methods has some impurities. To remove them, impure phosphorus is heated lightly with a dilute solution of acidic potassium dichromate. By doing this the impurities get oxidized. And exits as gas or a second layer forms at the bottom of the fluid.
Some phosphorus is also oxidized and destroyed. The remaining phosphorus is separated with the aid of an exit door at the bottom of the pot filled with fluid. And cool it. In this way pure phosphorus is obtained.
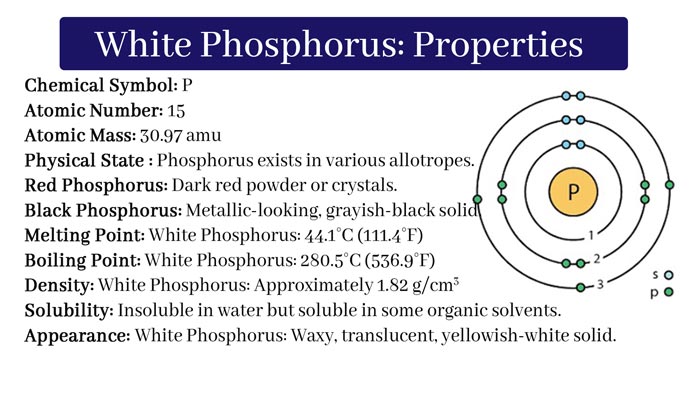
Physical Properties:
Ordinary phosphorus or white phosphorus is a soft solid. It can be sliced with a knife. It is colorless or white. But due to the effect of light, its color gradually turns yellow. It smells like garlic. Its melting point is 440C and the boiling point is 2870C. It is insoluble in water.
And soluble in Benzene, carbon disulfide and turpentine oil. By 10000C it has an atom of form P4. But at higher temperatures than it has Molecular Form P2. White phosphorus is a highly toxic substance. It is also fatal in low volume (0.15gm). When in contact with its vapor, it makes the bones strangled.
- The electron configuration of Elements
- How do you write a Chemical Formula?
- What is a molecule’s easy definition?
- NEET Exams 2020 cancelation, Admit card, Study for NEET 2020
- Chemicals in Medicine: Class 12
- Semiconductor Electronics: Materials, Devices, and Simple Circuits
Phosphorus is a more productive element. It reacts with many substances only at ordinary temperatures.
Sodium
3Na + P → Na3P
Calcium
3Ca + 2P → Ca3P2
Sulphur
3S + 2P → P2S3
Chlorine
2P + 3Cl2 → 2PCl3
2P + 5Cl2 → 2PCl5
Oxygen
4P + 3O2 → 2P2O3
4P + 5O2 → 2P2O5
When phosphorus is left open in the air, it reacts with oxygen of the air to form P2O3 and P2O5. Initially, this process of oxidation of phosphorus is slow. In this activity, heat is generated and temperature increases. After some time, when the temperature exceeds 300C, phosphorus starts burning. For this reason, do not keep it open in the air. But they keep it in water.
Energy is produced in its action with oxygen. And some part of the energy is released as green-yellow light. It glows when the inner phosphorus is kept open in the dark. This property of phosphorus is called phosphorescence.
Reaction with Acid:
It is oxidized to phosphoric acid when heated with concentrated sulfuric or nitric acid.
2P + 5H2SO4 → 2H3PO4 + 5SO2 + 2H2O
P + 5HNO3 → H3PO4 + 5NO2 + H2O
Alkali action:
This phosphine gas is formed when heated with a concentrated solution of alkalis.
4P + 3KOH + 3H2O → 3KH2PO2 + PH3↑
4P + 3NaOH + 3H2O → 3NaH2PO2 + PH3↑
Reducing properties:
It reduces the salts of copper, gold etc.
It reduces the copper sulfate in cold solution to copper and in hot solution to copper phosphide.
5CuSO4 + 2P + 8H2O → 5Cu + 2H3PO4 + 5H2SO4
3CuSO4 + 4P + 6H2O → Cu3P2 + 2H3PO3 + 3H2SO4
Structure of White Phosphorus:
A molecule of white phosphorus has four atoms. Each atom is sp3 hybridized and forms three single bonds with the other three phosphorus atoms. Thus a single electron pair is left on each phosphorus atom.
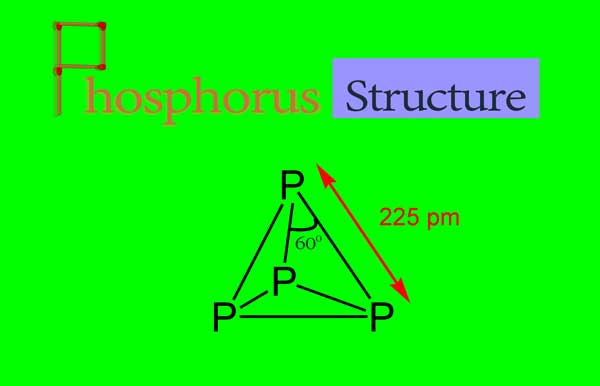
The structure of the white phosphorus molecule is tetrahedral. A phosphorus atom is located on the four vertices of the tetrahedron.
According to sp3 hybridization in the phosphorus molecule, the p -p -p bond angle should be around 1090 28’ while the bond angle is actually 600. Due to this the phosphorus molecule is strained. Due to this deformation, phosphorus is very active and reacts with many elements at ordinary temperatures.
Allotropy of Phosphorus
Phosphorus exhibits allotropy. White phosphorus is the major among them. Generally, white phosphorus is called phosphorus. The following is a brief description of other forms of phosphorus.
Red Phosphorus:
Red phosphorus is obtained by heating white phosphorus in the presence of iodine or any inert gas at about 2800C.
White phosphorus + I2 or other inert gas -2400C→ Red Phosphorus + 4.22Kcal
For the industrial manufacture of red phosphorus, the white phosphorus is mixed with a small amount of iodine and heated to about 2800C.
Thus, the majority of white phosphorus is converted to red phosphorus by heating. The mixture obtained is heated with a NaOH solution to separate red phosphorus from white phosphorus.

White phosphorus dissolves in NaOH as it is more reactive. And red phosphorus remains indestructible. After filtering this solution, wash the residue with water and dry it and get red phosphorus in a pure condition.
Sinduri Phosphorus
When white phosphorus is heated for 10 hours with a phosphorus tribromide solution, Sinduri phosphorus is obtained. It burns rapidly in the air like white phosphorus. In other properties, it is similar to red phosphorus.
α-Black Phosphorus
When red phosphorus is heated to 5300C in a closed drain, α-black phosphorus is obtained. It is not oxidized by oxygen of air at ordinary temperature.
β-Black Phosphorus
When white phosphorus is heated to 200 temperature and very high pressure, it turns into β-black phosphorus. It does not burn up to 400 in the air. Its structure is similar to that of graphite and it is the conductor of electricity.
Phosphorus Uses
Following are the major uses of phosphorus
Red phosphorus is used in making matches.
Phosphorus is used to make a useful Egyptian metal called Phosphor bronze. The Phosphor bronze consists of copper, tin, and phosphorus.
Phosphorus is used in making explosives, smoke screens, and fireworks.
Phosphorus is used to make some useful chemicals like hypophosphorous acid, phosphorus pentachloride, and Phosphoric Acid. hypophosphorous acid is also used in medicine.