Zinc Properties || What are the properties and uses of zinc?
Zinc
is a chemical element with the symbol Zn and atomic number 30. It is a bluish-white metal with a relatively low melting point and boiling point. In this article, we will explore the various properties of zinc, including its physical, chemical, and biological properties.
Zinc Preparation
Zinc is a common metal that is readily available and can be easily prepared through a variety of methods. Here are some of the most common methods for preparing zinc:
Electrolysis: One of the most common methods for preparing zinc is through the process of electrolysis. In this process, zinc oxide is dissolved in a solution of sulfuric acid and then an electric current is passed through the solution. This causes the zinc to be reduced to its elemental form, which can be collected at the cathode.
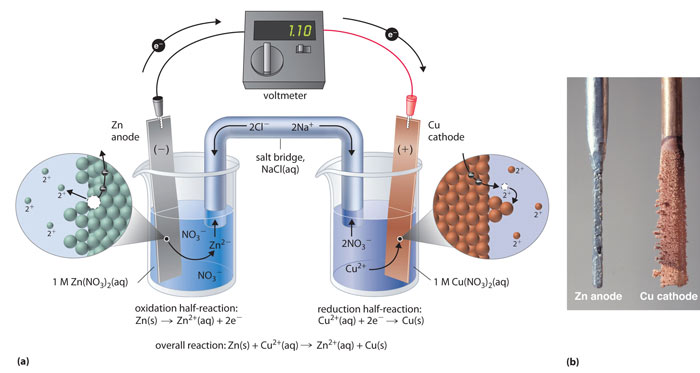
Image source : Click here
Smelting: Another common method for preparing zinc is through smelting. In this process, zinc ore is heated with carbon or coke in a furnace, which causes the zinc to be reduced to its elemental form. The resulting zinc vapor is then condensed and collected.
Roasting: Roasting is another method for preparing zinc from zinc ore. In this process, zinc ore is heated in the presence of oxygen, which causes the zinc sulfide to be converted to zinc oxide. The zinc oxide can then be reduced using one of the other methods, such as electrolysis or smelting.
Solvent extraction: Solvent extraction is another method for preparing zinc from its ores. In this process, a solvent is used to extract zinc from the ore. The resulting solution is then purified and the zinc is precipitated out of the solution using electrolysis or some other method.
Zinc dust: Zinc can also be prepared using zinc dust, which is a fine powder made from pure zinc. This powder can be used in various applications, including as a reducing agent and as a catalyst.
In general, the method used to prepare zinc will depend on the specific application and the availability of materials. Zinc is a widely used metal with many important applications in various industries, and as a result, there are many methods available for its preparation.
Zinc Properties
Zinc Physical Properties
Zinc is a moderately soft, ductile, and malleable metal that has a density of 7.14 g/cm³. It is a good conductor of electricity and has a melting point of 419.5°C (787°F) and a boiling point of 907°C (1,665°F). Zinc has a hexagonal crystal structure, and its atomic radius is 134 pm. It has five stable isotopes: 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn.
One of the most notable physical properties of zinc is its reactivity with oxygen. When exposed to air, zinc reacts with oxygen to form a layer of zinc oxide, which protects the metal from further oxidation. This layer of zinc oxide is also responsible for the characteristic bluish-white color of zinc.
Chemical Properties of Zinc:
Zinc is a highly reactive metal that readily reacts with other elements to form compounds. It has a valence of +2, which means that it can form ionic bonds with elements that have a negative valence. Zinc also forms covalent bonds with elements that have a similar electronegativity, such as carbon, nitrogen, and sulfur.
One of the most important chemical properties of zinc is its ability to form alloys with other metals. For example, brass is an alloy of copper and zinc, and it is widely used in plumbing fixtures, musical instruments, and decorative items. Zinc is also commonly alloyed with aluminum, magnesium, and other metals to improve their strength and corrosion resistance.
Biological Properties of Zinc:
Zinc is an essential nutrient for all living organisms, including humans. It plays a crucial role in many biological processes, including cell growth and division, DNA synthesis, immune function, and wound healing. Zinc is also required for the proper functioning of many enzymes, including those involved in the metabolism of carbohydrates, proteins, and fats.
Despite its importance in biological systems, excessive levels of zinc can be toxic. In humans, excessive zinc intake can lead to nausea, vomiting, and diarrhea. Long-term exposure to high levels of zinc can cause copper deficiency, which can lead to anemia and other health problems.
Applications of Zinc:
Zinc has many applications in various industries, including construction, automotive, and electronics. One of the most significant uses of zinc is in the production of galvanized steel. Galvanized steel is made by coating steel with a layer of zinc, which provides excellent corrosion resistance and durability. Zinc is also used in the production of brass, bronze, and other alloys.
In addition to its use in metal alloys, zinc is used in many other applications. For example, zinc oxide is used in the production of rubber, paint, and plastics. Zinc is also used in the production of batteries, including alkaline and zinc-carbon batteries.
Conclusion:
Zinc is a versatile and important element that has many applications in various industries. Its physical properties, including its low melting point and hexagonal crystal structure, make it a useful material for many applications. Its chemical properties, including its ability to form alloys and react with other elements, make it a key component in many manufacturing processes.
Its biological properties, including its essential role in many biological processes, make it a vital nutrient for humans and other living organisms. Overall, zinc is a valuable element with many important properties that make it an essential component of modern society.
