Crystal field theory || CFT for octahedral complexes
Crystal Field Theory (CFT) is a model used to explain the electronic structure and magnetic behavior of transition metal complexes. CFT considers the interactions between the transition metal ion and the surrounding ligand (or ion) field as a perturbation of the metal's electron configuration.
The perturbation causes splitting of the d-orbitals in the metal ion into two energy levels, producing a characteristic energy spectrum. The resulting electron distribution determines the color, magnetism, and reactivity of the complex.
CFT is a simple and useful model that provides a good starting point for understanding the properties of transition metal complexes in coordination chemistry.
This theory considers only the geometry of the d orbitals of a central cation and its interaction with some ligands considered as punctual negative charges.
According to this model, the ligands are attracted by the positive charge of the meta, but when approaching they generate repulsions on the d electrons of the cation, deforming the orbitals in which they are found.
A deformed orbital has a higher energy than one with its "natural" shape, so the electrons tend to occupy positions in the "native" orbitals whenever possible, this is whenever the difference in energy between the orbitals with the highest and the lowest.
Lowest energy is not less than the pairing energy due to the repulsion of electrons in the same orbital.
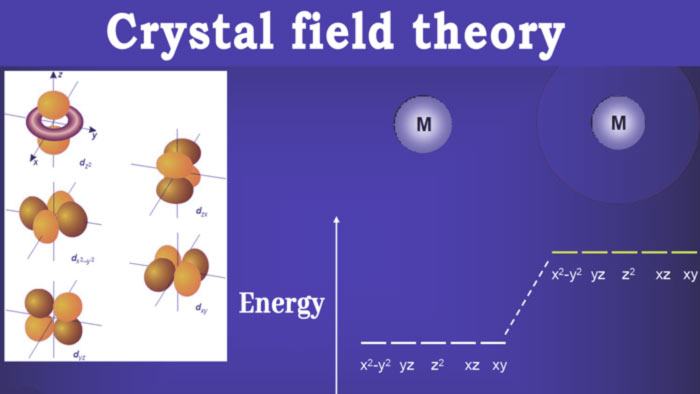
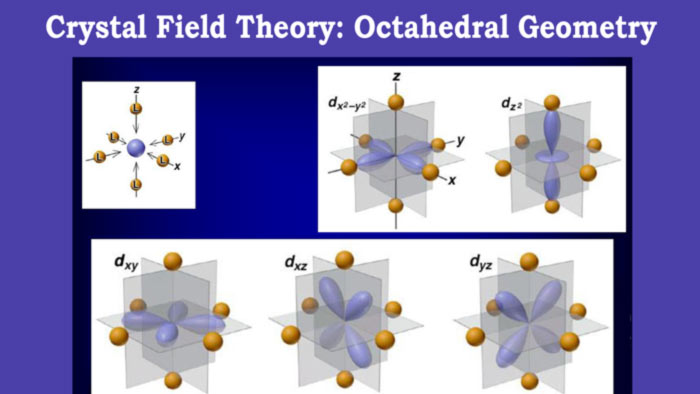
A simple example is what happens for an octahedral complex, if it is assumed that the ligands move along the axes of a three-dimensional Cartesian system, the orbitals that are going to be mainly affected are those with principal components on these axes.
looking at the graph of d orbitals you can see that these orbitals are d z2 and dx2-y2. As a consequence, they increase their energy and separate from the rest of the d orbitals, forming two subgroups of orbitals: the high energy group eg and the low energy group t2g.
The degree of separation between eg and t2g orbitals will depend on the strength of the ligands, that is, the degree to which these ligands are capable of deforming the d orbitals.
The spectrochemical series is an empirical table that orders the ligands according to the degree of separation they cause in the d orbitals, from lowest to highest strength they are:
I− < Br− < S2− < SCN− < Cl− < NO3− < N3– < F− < OH− < C2O42− < H2O < NCS− < CH3CN < py < NH3 < en < 2,2′-bipiridina < phen < NO2− < PPh3 < CN− < CO
According to this model, the electronic transitions between these d orbitals of different energy are responsible for the absorptions of certain wavelengths that give color to the complexes (the shorter the wavelength, the higher the energy and therefore the greater the strength of the ligand).
When the separation between low-energy and high-energy orbitals is greater than the pairing energy of the electrons, all the electrons tend to occupy positions in the low-energy orbitals according to the Aufbau principle, forming what is known as a low spin complex.
On the other hand, if the separation between orbitals is smaller than the pairing energy, the electrons tend to occupy all the orbitals (whether low or high energy) before starting to pair according to Hund’s rule, forming what is known as a high spin complex.
According to this model, the number of electrons in paired or unpaired spin conditions are responsible for the magnetic properties of the complexes.
The important point of CFT:
- This theory considered the metal-ligand bond is purely ionic
- The electrostatic interaction between metal ion and ligands
- The ligand is anion metal atom is a cation
- If the ligand is a neutral molecule the negative ends of the dipole is attractive
towards the metal atom
- If the ligand is a neutral molecule the negative ends of the dipole is attractive
- It treats the ligand is a point of negative charge
- The arrangement of ligand around the central metal ion in such a way that repulsion should be minimum