Electrolysis : Definition, Principle and Electrolytic Cell
Electrolysis is a process in which electric current is
used to force a redox reaction. Electrical energy is converted into chemical
energy.
For example, electrolytic cells are used for the
production and purification of substances.
Electrolysis takes place in an electrolysis cell. This is in contrast to the galvanic cell. Using the example of a rechargeable battery, you can understand this from: The charging process is electrolysis, the discharging process is the galvanic reaction.
Electrolysis Definition
Electrolysis is a redox reaction forced by electrical energy. The electrical energy is converted into chemical energy.
Electrolysis has a wide range of applications in chemistry: for example in the extraction of metals (aluminium) or the splitting of water into hydrogen and oxygen.
Electrolytic Cell
The electrolysis takes place in an electrolysis cell. A chemical reaction is forced by the inflow of electrical energy.
Structure of the Electrolytic Cell
An electrolytic cell consists of two electrodes on which the partial reactions of the redox reaction take place. The electrodes are connected across a voltage source and are immersed in a conductive liquid. You also refer to the conductive liquid as an electrolyte. It consists of cations and anions.
The electrode connected to the negative pole is called the cathode and the electrode connected to the positive pole is called the anode.
Accordingly, the structure of electrolytic cells and galvanic cells are very similar. But there are two fundamental differences:
the electrolytic cell uses a voltage source, the galvanic cell uses a current collector
electrolysis takes place in a container, the galvanic cell is divided into two
Electrolysis Principle
Electrolysis works as follows: If you apply direct current across the two electrodes, the electrons flow in the direction of the cathode. As a result, there is a shortage of electrons at the anode and an excess of electrons at the cathode.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- IUPAC Name : How to find the IUPAC name of compounds.
The excess of electrons at the cathode causes the positive cations to move to the cathode. There they absorb the excess electrons and are thus reduced. Meanwhile, the same number of negative anions migrate to the positive anode and give up their electrons there (oxidation).
A minimum voltage is required for electrolysis to take place. You also call it the decomposition voltage (Uz). Only when your decomposition voltage is reached does the electrolysis take place.
Electrolysis Examples
There are many examples of electrolysis. So that you can better understand the principle of electrolysis, we will show you a few. The electrolyses of…
zinc iodide (ZnI2)
Sodium chloride (NaCl) and water
Electrolysis zinc iodide
Zinc iodide (ZnI2) is a chemical compound of zinc and iodine and is one of the halides. You can use an aqueous solution of zinc iodide as the electrolyte.
If you now apply a DC voltage, the positively charged zinc ions (Zn2+) migrate to the cathode. There they absorb two electrons (reduction) and elemental zinc (Zn) is formed at the cathode. A reverse reaction of the elementary zinc is prevented by a uniform decomposition voltage and thus a constant excess of electrons at the cathode.
The negatively charged iodide ions (2I-) collect at the anode. There they each give up an electron (oxidation). This produces elementary iodine (I2).
Oxidation: 2 I– -> I2 + 2e–
Reduction: Zn2+ + 2e– -> Zn
You can represent the entire redox reaction as follows:
Redox reaction: Zn2+ + 2 I– -> Zn + I2
As you can see, the action of the electric current has broken the chemical compound zinc iodide into the elements zinc and iodine.
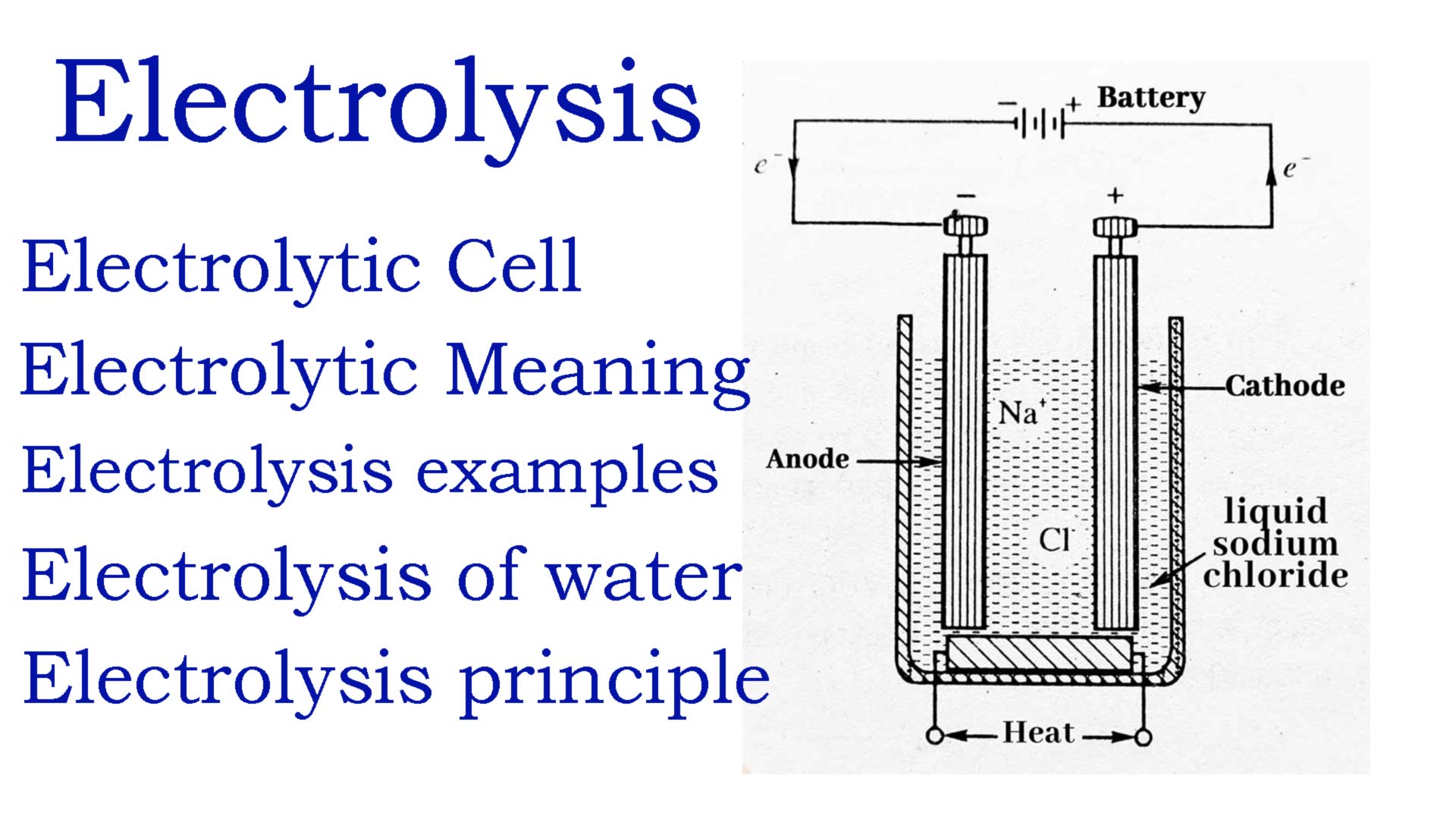
Electrolysis sodium chloride
You also call the electrolysis of sodium chloride common salt electrolysis. Sodium chloride is decomposed by an electric current. It belongs to the chloralkali electrolysis. You use an aqueous solution of sodium chloride as the electrolyte.
The excess of electrons at the cathode causes the protons (H+) present in the water to absorb an electron (reduction). This produces hydrogen (H2). In addition, hydroxide ions are formed, which then go into solution.
The negatively charged chlorine ions (Cl–) collect at the anode and give off an electron (oxidation). Two chlorine atoms then form a chlorine molecule (Cl2).
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- IUPAC Name : How to find the IUPAC name of compounds.
The free sodium cations (Na+) together with the remaining hydroxide ions (OH–) form a diluted caustic soda.
The reaction equation is:
2 NaCl + 2 H2O → 2 NaOH + H2 + Cl2
Electrolysis of water
By electrolysing water, you can even break water down into its constituents, hydrogen (H2) and oxygen (O2). The water electrolysis consists of two different partial reactions at the two electrodes.
The reaction equations at the cathode and the anode are as follows:
Cathode: 2 H2O + 2e– → H2 + 2 OH–
Anode: 2 H2O → O2 + 4H+ + 4e–
Redox reaction: 2 H2O → 2 H2 + O2

Electrolysis Efficiency
Basically, electrolysis is a very energy-efficient process with an efficiency of over 70%. It is important that you choose a voltage close to the decomposition voltage for the process.
In order to make the electrolysis of gases more reliable, a certain amount of extra voltage is required. This “overvoltage” is strongly dependent on the electrode material and often also reduces the efficiency of the reaction.
A diaphragm can also impede the flow of electrical current. As a result, the efficiency of the entire electrolysis process can be reduced.
Electrolysis application areas
Electrolysis is used in particular for a wide variety of technical material separations. But it can also be used for metalworking and wastewater treatment. In the following we describe three important areas of application of electrolysis.
chlor-alkali electrolysis
With chloralkali electrolysis you can produce the products chlorine, hydrogen and caustic soda from an aqueous solution of sodium chloride. You can distinguish between three technical processes:
In the diaphragm process, the cathode and anode chambers are separated by an asbestos diaphragm. The cathode is usually made of steel and the anode is made of coated titanium. The anode and cathode reactions are composed as follows:
Anode: 2Cl–(aq) → Cl2(g) +2e–
Cathode: 2 H3O+(aq) + 2e– → OH–(aq) + H2(g)
The free sodium ions (Na+) together with the resulting hydroxide ions (OH–) form caustic soda (NaOH) up to a concentration of approx. 15%.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- IUPAC Name : How to find the IUPAC name of compounds.
The second method is the so-called membrane method. Here, the diaphragm is replaced by a thin, chlorine-resistant membrane. As with the diaphragm process, the anode is made of titanium, but the cathode is made of nickel. The chemical reactions correspond to those of the diaphragm process.
As a third and final method of chlor-alkali electrolysis, you can use the amalgam process. This consists of a titanium anode and a mercury cathode. The sodium formed at the cathode dissolves in the mercury as sodium amalgam. The amalgam process also gets its name from this reaction.
Theoretically, the following electrode reactions can take place:
Anode:
2 Cl–(aq) → Cl2(g) + 2e–
4 OH–(aq) → O2(g) + 2H2O +4e–
Cathode:
Na+(aq) + e− → Na
2H3O+(aq) + 2e– → H2 + 2H2O
The sodium reacts immediately at the mercury cathode to form sodium amalgam.
Na/Hgx
If you now want to get caustic soda from the reaction, then your sodium amalgam must be reacted in another chamber, the amalgam decomposer. By reacting with water, the sodium malgane decomposes into caustic soda:
2 Na/Hgx + 2H2O → 2 NaOH(aq) + H2(g)
As an overall reaction you get:
2 NaCl(aq) + 2H2O → 2NaOH(aq) + Cl2(g) + H2(g)
Kolbe electrolysis
In Kolbe electrolysis, carboxylic acids or their salts (carboxylates) are split. The goal of this reaction is to couple two residues together. First, radicals are formed, which can then react further to form various hydrocarbons. For example, the discoverer of the reaction, Herrmann Kolbe, was able to produce ethane from acetic acid.
Electroplating
In electroplating, or electroplating, electrolysis is used to deposit metals. Since the quality of a workpiece often depends on its metallic luster, the effectiveness of the electrolysis is particularly important here.
Galvanic cell
You have now got to know the structure, the principle and some examples of electrolysis. You can use electricity to force a chemical reaction. However, chemical reactions in galvanic cells can also deliver electricity in reverse. Check out our post now to learn more about it!