What is aluminum bromide and how is it used in industry?
What is Aluminum Bromide ?
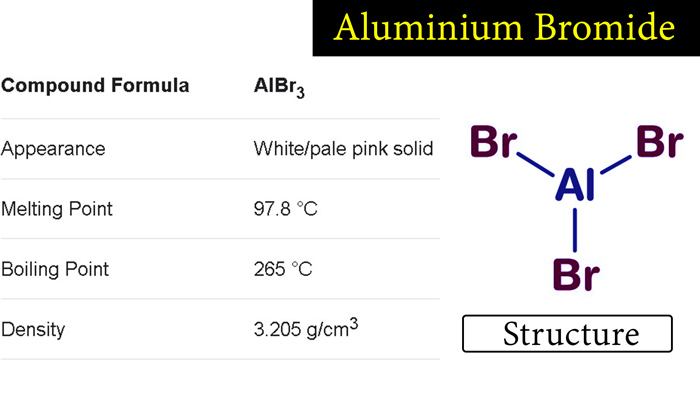
Aluminum bromide is an inorganic compound with the chemical formula AlBr3. It is a white, hygroscopic solid that is soluble in water and polar organic solvents. Aluminum bromide is a Lewis acid, meaning that it can accept electron pairs from other molecules to form a new bond.
As a result, it is commonly used as a catalyst in organic reactions, particularly in the Friedel-Crafts reaction for the synthesis of aromatic compounds.
It can also be used as a component in the production of aluminum metal and as a flame retardant. However, it is important to handle aluminum bromide with care as it is a corrosive and toxic substance.
Being a compound joined by a metal (aluminum) and a non-metal (bromine), covalent bonds are formed that give the structures a very good stability, but without reaching that of an ionic bond.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- IUPAC Name : How to find the IUPAC name of compounds.
Aluminum bromide is a substance that normally occurs in a solid state, with a crystalline structure. The colors of the different aluminum bromides appear as pale yellows of various shades, sometimes with no apparent color.
The color depends on the ability of the compound to reflect light and changes depending on the structures that are created and the shapes it takes.
The solid state of these compounds crystallizes, so they have well-defined structures that look similar to sea salt, but vary in color.
Formula for Aluminum bromide
Aluminum bromide is made up of an aluminum (Al) atom and different amounts of bromine (Br) atoms, depending on the number of valence electrons aluminum has.
Therefore, the general formula for aluminum bromide can be written as follows: AlBrx, where “x” is the number of bromine atoms that are attached to aluminum.
The most common form in which it occurs is as Al2Br6, which is a molecule with two aluminum atoms as the main bases of the structure.
The bonds between them are formed by two bromines in the middle, so that each aluminum atom has four bromine atoms in its structure, but in turn, they share two.

Properties and characteristics of aluminum bromide
Due to its nature, it is highly soluble in water, but it is also partially soluble in compounds such as methanol and acetone, unlike other types of substances.
It has a molecular weight of 267 g/mol and is formed by covalent bonds.
Sodium bromide reaches its boiling point at 255 °C, and reaches its melting point at 97.5 °C.
Another characteristic of this compound is that it emits toxins when it evaporates, so it is not recommended to work with it at high temperatures without proper protection and relevant safety knowledge.
Use of Aluminum Bromide
One of the uses given to this type of substance, due to its metallic and non-metallic nature, is as agents in chemical purity tests. These purity tests are very important in determining the quality of reagents and making products that people are satisfied with.
In scientific research it is used very variably. For example, to form complex structures, agents in the synthesis of other valuable chemical products, in the hydrogenation of dihydroxynaphthalenes and in selectivity in reactions, among other uses.
This compound is not popular commercially. As seen above, it has some applications that are very specific, but very interesting for the scientific community.