Hybridization : Definition, Meaning, Types with Examples
Hybridization Definition
Hybridization is the process of forming the same number of similar orbits from two or more unequal orbits of an atom.
in other words
“Mixing and redistribution of energy among atomic orbitals is known as Hybridisation.”
When two or more orbitals are hybridized, the number of orbitals obtained from hybridization is equal to the number of orbitals used in hybridization and the energy of the new orbitals is equal to each other.
Carbon has four electrons in its outer shell and its valency is also four. In most compounds, carbon forms four covalent bonds. Its electronic configuration in ground state is 1s2, 2s2, 2p1x, 2p1y.
- Hybridization : Definition, Meaning, Types with Examples
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
According to the modern concept of covalent bonding, a covalent bond is formed by the overlapping of two half-filled orbits. Hence the electronic configuration of carbon in excited state to form four covalent bonds becomes as follows –

Observing the electronic configuration of carbon, it appears that if carbon forms four single bonds, they will not be identical. In fact, if carbon forms four single bonds, then these four bonds are the same.
Hence it is assumed that the four orbits (s and p) of the outer shell of carbon form four new orbitals by hybridization which are similar to each other.
one s orbital + 3 p orbital → 4 sp3 hybridised orbital
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
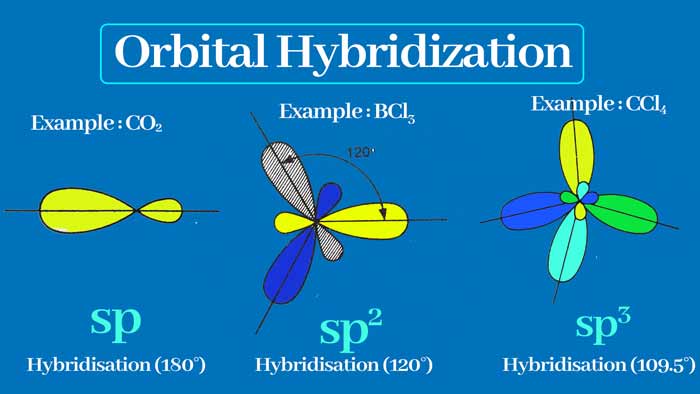
Similarly other types of hybridization are also possible in carbon. In sp2 hybridization one s and two p orbitals form 3 sp2 hybridization orbit.
In sp hybridization, one s and one p orbit form two sp hybridization orbits. Like carbon, some other elements also have a hybridization of the orbitals of the atoms.
Hybridization Types
sp3 Hybridization :
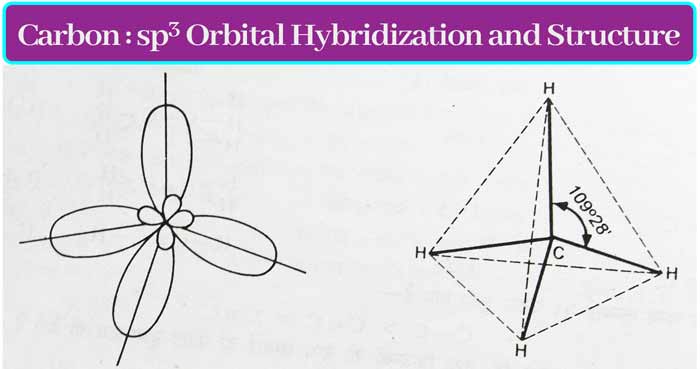
When one s orbital and 3 p orbitals are hybridized, then four new orbitals are formed which are called sp3 orbital and this type of hybridization is called sp3 hybridization.
1 s orbital + 3 p orbital → 4 sp3 orbital
The covalency of carbon is four. When these four bonds are single bonds, sp3 hybridization occurs in the carbon. The sp3 orbits have an angle of 109° 28’ and are directed towards the four vertices of a regular tetrahedron.
Example:
Methane CH4: The hybridization of the carbon atom in methane is of sp3 type. A σ bond is formed by the overlap of each sp3 orbital and the s orbital of H. Thus the shape of methane molecule is regular tetrahedron.
Like methane, CCl4, CHCl3, CH2Cl2, C2H6, C2H5OH, etc. In many other carbonic compounds where only single bonds are formed with carbon, the hydridization of carbon occurs in a similar manner.
Silane SiH4 : Like carbon, silicon also has four electrons in its valence shell. Like methane, there is sp3 hydridization of Si in SiH4 and its shape is also a regular tetrahedron.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
Ammonia NH3 : The electronic configuration of nitrogen is 1s2,2s2 ,2px1, 2py2, 2pz1. If the px, py and pz orbitals of N are used to overlap, then the bond angle in NH3 should be 90°.
In NH3 each N – H bond makes an angle of 107° with the other N – H bonds and its shape is Pyramidal. This fact is explained by the sp3 hydridization of N.

The sp3 hybridization of N gives four sp3 orbitals. One of these sp3 orbitals is completely filled and is not used for bonding. The remaining three sp3 orbitals are used to form bonds with hydrogen.
The shape of sp3 hybridization orbitals is a regular tetrahedron.
Since NH3 does not have an sp3 orbital of N in bonding, the shape of NH3 is Pyramidal.
According to VSEPRT(Valency shell Electron Pair Repulsion Theory), the repulsion between lone and bonding pairs of electrons is as follows:
Lone pair – lone pair > lone pair – bond pair > bond pair – bond pair
According to this principle, the angle between the N – H bonds in the NH3 molecule is narrowed. Hence, the bond angle in NH3 is 107°(Less than 109°28’).
Water H2O : The electronic configuration of oxygen is 1s2, 2s2, 2px2, 2py1, 2pz1. As a result of sp3 hybridization of oxygen, four sp3 orbitals are obtained. Two of these orbitals are complete and are not used for bonding. The remaining two orbitals are used to form bonds with hydrogen.
Since a molecule of water has only two bonds, it has an angular shape.
According to the VSEPR principle, the contraction in the angle between the bonds in H2O will be greater than that of NH3. Hence the bonding angle in H2O is 105°.
- Hybridization : Definition, Meaning, Types with Examples
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
When water and H+ combine to form H3O+ or ice is formed when the number of hydrogen bonds of water increases, the orbits of oxygen are composed of a regular tetrahedron.
sp2 Hybridization :
When there is a hybridization of one s orbit and two p orbitals, then three new orbitals are formed which are called sp2 orbital and this type of hybridization is called sp2 hybridization.
The covalency of carbon is four. When it forms three sigma and one pi bond, it undergoes sp2 hybridization. There is an angle of 120° between the sp2 orbits and the shape of the related molecule is plannar trigonal.
1 s orbital + 2 p orbital → 3 sp2 orbital
Example:
EthyleneC2H4 : C2H4 In ethylene each carbon atom is sp2 hybridized. So there are three sp2 orbital and ek pz orbital on each carbon atom which are used to make bond as follows –
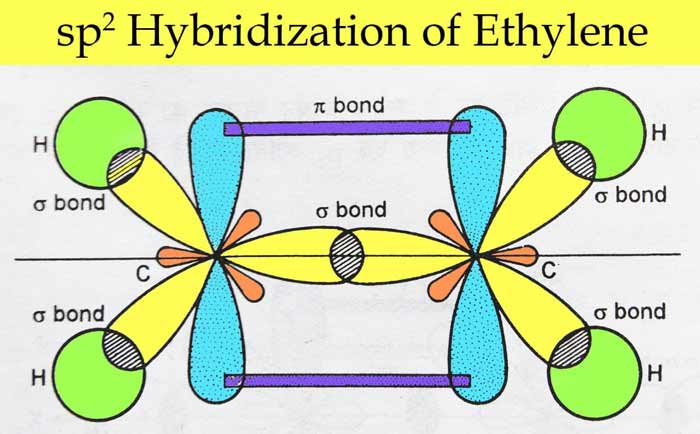
Boron tri fluoride(BF3) – The electronic configuration of boron is 1s2, 2s2, 2px1. In excited state, its electronic configuration becomes 1s2, 2s1, 2px1, 2py1. In this state, three sp2 hybridized orbitals are obtained as a result of hybridization, which form a sigma bond by linear overlap with the p orbital of each F atom.
sp Hybridization:
When an s orbital and a p orbital are hybridized, two new orbitals are formed which are called sp orbital and this type of hybridization is called sp hybridization.
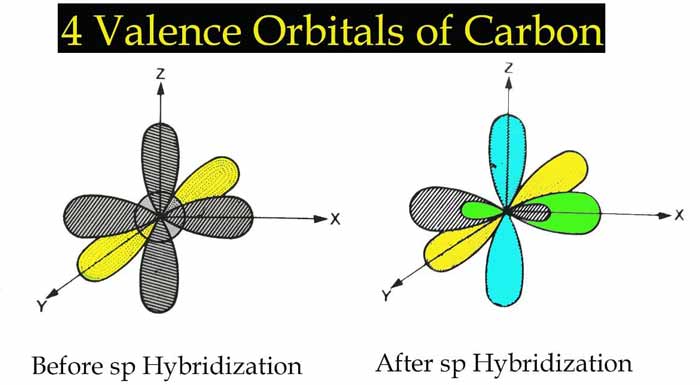
1 s Orbital + 1 p Orbital → 2 sp orbital
When an s orbital and a p orbital are hybridized, two new orbitals are formed which are called sp orbital and this type of hybridization is called sp hybridization.

The shape of each sp orbital shows that it has both s and p characters.
There is an angle of 180° between sp orbitals. Therefore, there will be an angle of 180° between the bonds formed with sp orbitals and the shape of the related molecule will be linear.
Example:
Acetylene C2H2 :
The hybridization of each carbon atom in acetylene is of sp type. Therefore, on each carbon atom there are two sp orbital, one py orbital and one pz orbital which are used to form bond as follows –

Beryllium hydride (BeH2):
The electronic configuration of berilium is 1s2, 2s2. In excited state its configuration becomes 1s2, 2s1, 2px1. In this state, falswarup of sp hybridization, two sp hybridized orbitals are obtained, which are used to form bonds in this way –

Other types of Hybridization:
Atoms of many elements have d-orbitals. Atoms of these elements can participate in the formation of d orbital bonds. Atoms of these elements can also participate in d-orbital hybridization.
Example:
In [NiCN4]2-, Ni atom is dsp2 hybridized and its shape is square planar. In PCl5, the P atom is sp3d hybridized and its shape is trigonal bipyramidal.
In SF6, the S atom is sp3d2 hybridized and its shape is octahedral. In IF7, the I atom is sp3d3 hybridized and has a pentagonal bipyramidal shape.
Hybridization : Definition, Meaning, Types with Examples
Preparation of Aldehydes and Ketones : Chemistry Page
Arsenious Oxide : Preparation, Properties and Uses
How to find Equivalent Weight in Chemistry ? Chemical Formula
Oxidation Number : How to find Oxidation State
If the central atom in a molecule is an element of s or p block, then the information about its type of hybridization and the shape of the molecule is obtained as follows –
First we write down the basic state of the central atom, its electronic configuration and the bonding formula of the molecule. On this basis the number of sigma bonds formed by the central atom is determined.
Here it will be useful to remember that a double bond has one sigma bond and a covalent bond also has only one sigma bond. After this, the number of lone electron pairs present on the central atom is equal to the sum of the number of lone electron pairs located on it.
Example: To find the hybridization of S in SO2 and the shape of SO2, write the electronic configuration of S and the bonding formula of SO2 –

In SO2 molecule, S forms two sigma bonds and a lone electron pair is present on S. Hence the three orbitals of S participate in the hybridization. So in SO2 the S atom will be sp2 hybridized. In sp2 hybridization the shape of the molecule is square trigonal.
Since SO2 has one sp2 hybridized orbital used by a lone electron pair and not used for bonding, SO2 will have an angular shape.

Number of sigma bonds formed by Cl = 3
Number of lone electron pairs located on Cl = 1
Number of orbitals used by Cl in hybridization = 3 + 1 = 4
So in ClO3 the Cl atom will be sp3 hybridized. In sp3 hybridization the shape of the molecule is regular tetrahedron but because one vertex of the tetrahedron is used by a lone electron pair, the shape of the molecule will be pyramidal.