Oxidation Number : How to find Oxidation State
Oxidation Number or Oxidation state
The charge present on an atom or group of atoms is called the oxidation number or Oxidation State of that atom or group of atoms.
Example:
sodium chloride (NaCl) has a +1 charge on the sodium atom and -1 charge on the chlorine atom. Therefore, in sodium chloride, the oxidation number of Na is +1 and that of Cl is -1.
Similarly in Al2O3 the oxidation number of Al is +3 and the oxidation number of O is -2.
The oxidation number of atoms or groups of atoms on which no real charge is present is determined according to the following rules and these are called oxidation number rules or oxidation state rules :
oxidation number rules
A) If a covalent bond is present between two ether atoms, then both the shared electrons are counted in the atom which has the highest electronegativity.
Example:
The shared electrons between I and Cl in I – Cl are both counted in chlorine, so chlorine in I – Cl has the oxidation number -1 and the oxidation number of iodine is +1.
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
B) If a covalent bond is present between two similar atoms, then one electron in one atom and another electron in the other atom are counted from the two shared electrons.
Example:
In H – H, one of the two electrons shared between the two hydrogen atoms is counted in one hydrogen atom and the other electron in the other hydrogen atom, so the oxidation number of H in H – H is zero.
Thus by writing the electronic center formula of any compound or ion, the oxidation number of any part of it can be found.
Actually this method takes more effort and time, so to find the oxidation number of different atoms and groups of atoms conveniently, some other rules have been made with the help of above rules which are as follows.
With the help of the following rules, the oxidation number of any part of a compound or ion can be found conveniently –
1) The oxidation number of all elements in the free state is zero. In other words, the oxidation number of atoms of one element is zero if they are not attached to atoms of another element.
Example:
The oxidation number of hydrogen atoms in hydrogen molecule (H – H) is zero. Similarly, the oxidation number of these elements is zero in the molecules of nitrogen (N2), chlorine (Cl2) bromine (Br2) phosphorus (P4) etc.
2) Almost all compounds of hydrogen have an oxidation number of +1. It has an oxidation number of -1 only in the hydrodon of metals (example: LiH , NaH , CaH2).
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
3) Oxygen has an oxidation number of -2 in most compounds. Oxygen in peroxides, super oxides, ozonides and some other compounds does not have an oxidation number of -2. In peroxides there is a single bond between two oxygen atoms. And in these, the oxidation number of oxygen is -1, in F2O the oxidation number of O is +2.
4) In the compounds of 4 alkaline metals (Li, Na, K etc.), they have an oxidation number of +1.
5) In the compounds of 5 alkaline earth metals (Be , Mg , Ca , Sr , Ba and Ra ) they have an oxidation number of +2.
6) Fluorine has an oxidation number of -1 among all its compounds. The oxidation numbers of chlorine, bromine and iodine are also -1 in most of their compounds.
7) All compounds have an oxidation number of zero. Therefore, the sum of the oxidation numbers of the atoms present in a molecule of a compound is also zero.
example :
The oxidation number of NaCl is zero, the oxidation number (+1) of sodium and the oxidation number (-1) of chlorine will also add up to zero.
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
8) The oxidation number of an ion is equal to the charge on it.
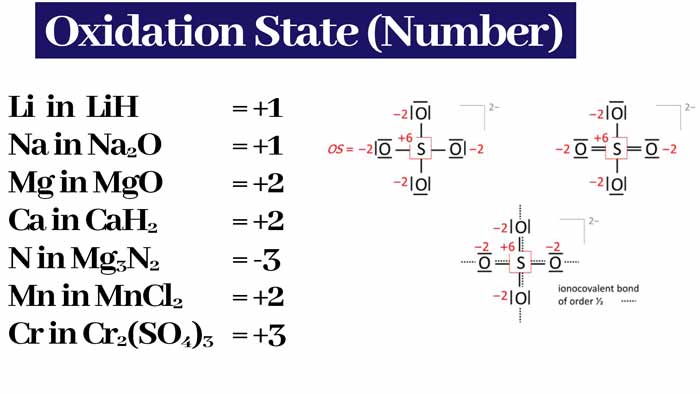
Example : The oxidation numbers of Cl–, SO42-, PO43- and Al3+ ions are -1 -2 -3 and +3 respectively.
9) The sum of the oxidation numbers of the atoms present in an ion is equal to the oxidation number of that ion.
Example : SO42- oxidation number of -2 is . The oxidation number of the oxidation numbers present in it is also -2. Assuming the oxidation number of the sulfur atom present in it to be X –

-2 = X + 4 x (-2)
so X = +6
So the oxidation number of sulfur in SO42- is +6.
10) If more than one atom of the same element is present in one molecule or one ion of a compound, then the oxidation number of that element in that compound or ion is equal to the average of the oxidation numbers of all the atoms of that element.
Example: n-propane has the molecular formula C3H8 and the structural formula CH3 – CH2 – CH3. It has three carbon atoms in one molecule. whose oxidation numbers are -3 , -2 and -3 respectively. So the oxidation number of C in C3H8 is -8/3.
11) The value of the oxidation number can be zero, positive, integer or fraction.
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
Oxidation State
The oxidation number of an atom of an element is also called its oxidation state. Hence, the term Oxidation State is used for an atom of an element whereas the term Oxidation number is also used for any one or a group of atoms of different elements.
Example: In C3H8, the oxidation number of C is -8/3 while the oxidation states of carbon atoms are – 3, -2 and -3 respectively.
Obviously, the oxidation number can be fractional, while the oxidation state is always a whole number.
Difference between oxidation number and valency :
The valency of an element is equal to the sum of the number of valid and covalent bonds formed by one of its atoms. The oxidation number (or oxidation state) of an element is the charge present on one of its atoms in a compound of that element.
The valency of an element is always in integer. The oxidation number of an element is usually an integer. But in some cases it can also be fractional.
example :
The oxidation number of S in Na2S4O6 is +2.5. The value of the valency of an element is always positive and the plus (+) sign is not used with it. The oxidation number of an element can be positive or negative and must be accompanied by a plus (+) or minus (-) sign.
In some cases the numerical values of the oxidation number and valency of the elements are equal.
In some cases the numerical values of the valency and oxidation number of the elements are different.
Example: In CH2Cl2 the valency of carbon is four and the oxidation number is zero.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
oxidation number example:
Q. Find the oxidation number of O in O3.
Ans: O atoms in O3 do not form bonds with atoms of any other element. In the free state, the oxidation number of all elements is zero. So in O3 the oxidation number of O will be zero.
Q. Find the oxidation number of Ba in BaCl2 .
Ans: BaCl2 is an ionic compound. In this, there is a valid covalent bond between one Barium atom and two chlorine atoms. In this, two units of barium are positive and one unit is negative for each chlorine atom. So in BaCl2 the oxidation number of Ba will be +2.