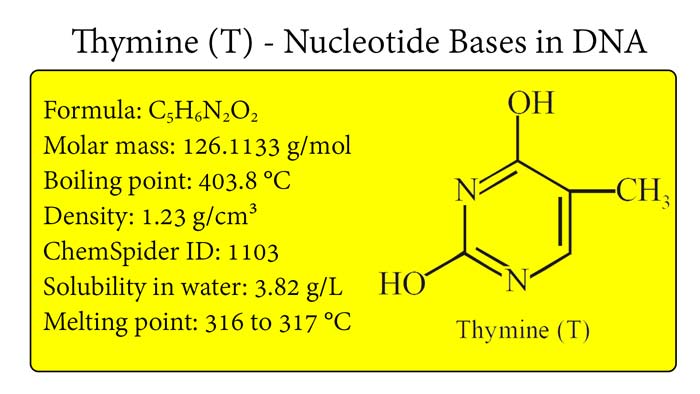
Thymine : Properties, Thymine in DNA, C5H6N2O2
Thymine is a nitrogen-containing base that is one of the four nucleotide bases that form the genetic code of DNA (deoxyribonucleic acid). Thymine is a pyrimidine base, meaning that it has a six-membered ring structure containing two nitrogen atoms and four carbon atoms. In this article, we will discuss the chemical and physical properties of thymine.
Chemical Properties of Thymine
Thymine has a molecular formula of C5H6N2O2 and a molecular weight of 126.11 g/mol. The nitrogen atoms in thymine have lone pairs of electrons, which can form hydrogen bonds with the complementary base adenine in the DNA double helix. Thymine can form two hydrogen bonds with adenine, while guanine can form three hydrogen bonds with cytosine.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
Thymine can undergo a process called tautomerization, where the hydrogen atoms on the nitrogen atoms switch positions. This can lead to the formation of a rare base called 2-hydroxy-4-aminopyrimidine, which can cause mutations in DNA if it is incorporated into the genetic code.
Thymine can also undergo oxidation reactions, where the double bonds in the pyrimidine ring are broken. This can lead to the formation of thymine glycol, a modified base that can cause DNA damage if it is not repaired by the cell’s DNA repair machinery.
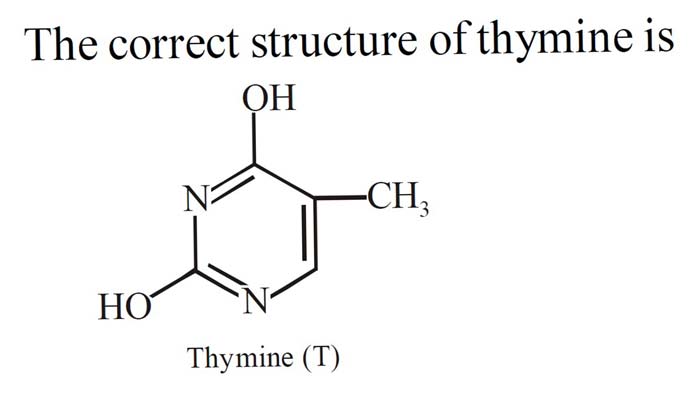
Chemical Properties of Thymine
Thymine is a white or colorless crystalline solid that is soluble in water and organic solvents such as ethanol and acetone. Thymine has a melting point of 316-317 °C and a boiling point of 428-429 °C.
Thymine has a UV absorption maximum at 267 nm, which can be used to detect and quantify thymine in biological samples. Thymine is also fluorescent, with a maximum emission at 440 nm, which can be used to visualize thymine in DNA or RNA.
Thymine is relatively stable under normal physiological conditions, but it can be damaged by exposure to UV radiation. UV radiation can cause the formation of cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts in DNA, which can interfere with DNA replication and transcription.
Thymine in DNA:
Thymine is one of the four nucleotide bases that make up the genetic code of DNA. Thymine always pairs with adenine, forming two hydrogen bonds between the nitrogen atoms of the bases. This base pairing is essential for the stable and accurate replication of DNA during cell division.
The sequence of nucleotide bases in DNA determines the genetic information that is passed from parents to offspring. Mutations in the DNA sequence, such as those caused by tautomerization or oxidative damage to thymine, can lead to changes in the genetic code that can affect protein function and contribute to diseases such as cancer.
Conclusion:
Thymine is a nitrogen-containing base that is one of the four nucleotide bases that form the genetic code of DNA. Thymine is a pyrimidine base with a six-membered ring structure containing two nitrogen atoms and four carbon atoms. Thymine can form two hydrogen bonds with adenine in the DNA double helix.
Thymine is relatively stable under normal physiological conditions, but it can be damaged by exposure to UV radiation, leading to the formation of DNA damage such as CPDs and (6-4) photoproducts. Mutations in thymine can lead to changes in the genetic code that can affect protein function and contribute to diseases such as cancer.