Alkenes : Aliphatic Unsaturated Hydrocarbons
Those compounds are called Aliphatic Unsaturated Hydrocarbons :-
1 – Aliphatic are those in which all the chains of atoms present are open.
2 – Those that are unsaturated i.e. in which at least one carbon-carbon double bond or carbon-carbon triple bond is present.
3- In which only carbon and hydrogen elements are present. These aliphatic unsaturated hydrocarbons are called alkenes in which only one carbon-carbon double bond is present.
Those aliphatic unsaturated hydrocarbons are called alkynes in which only one carbon-carbon triple bond is present.
What is Alkenes?
Those aliphatic unsaturated hydrocarbons are called alkenes in which only one carbon-carbon double bond is present. The general formula of alkenes is CnH2n and all alkenes belong to the same homologous series. Substituting the value of n as 1 in the general formula CnH2n, the formula obtained is CH2. In this formula all four valencies of carbon are not satisfied.
Hence it does not show any compound. It exhibits a bivalent free radical named Ethylene (C2H4) as the simplest member of the series. This compound reacts with chlorine to form an oil-like substance called Ethylene dichloride. Hence the members of this category are also called Olefins. The double bonds present in olefines are also called Olefinic bonds.
Nomenclature of Alkenes :
In the IUPAC method, the nomenclature of alkenes is done by removing ene from the end of the name of the alkane and adding ene. Following are the molecular formula, structure formula, common name IUPAC name of some major alkenes –
| FORMULA | STRUCTURE FORMULA | NAME | IUPAC NAME |
|---|---|---|---|
| C₂H₄ | H₂C=CH₂ | Ethylene | Ethene |
| C3H6 | CH3CH=CH2 | Propylene | Propene |
| C4H8 | CH₃CH₂CH=CH₂ | 1-Butylene | 1-Butene |
| C4H8 | CH3-HC=CH-CH3 | β-Butylene | 2-Butene |
| C4H8 | (CH3)2C=CH2 | Isobutylene | 2-methylpropene |
Isomerism in Alkenes :
The first two members of the alkene series (ethylene and propene) do not show isomerism. The other members of this category represent Place Isomerism, Series Isomerism and Geometric Isomerism.
example :
The molecular formula C4H8 shows four isomerism of alkenes –

Of these four isomerisms, I and II are series isomerism, II and III are series isomerism, II and IV are series isomerism, I and III are space isomerism, I and IV are space isomerism and III and IV are geometric isomerism.
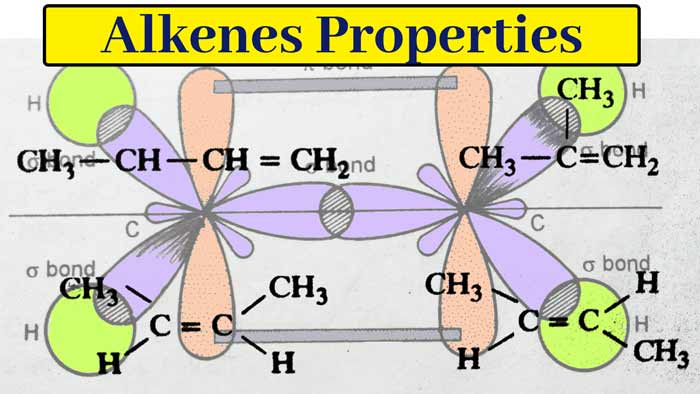
Structure of Alkenes
The structure of alkenes can be explained by the example of ethylene. In ethylene (C2H4) each carbon atom is sp2 hybridized. So each carbon atom has three sp2 hybridized orbitals and one pure p orbital. Three sp2 hybridized orbitals are used to form three sigma bonds. The remaining one p orbital on both carbon atoms form a pi bond by lateral overlap.

Thus ethylene molecule has one carbon-carbon double bond and four carbon-hydrogen single bond. In other words ethylene molecule has 5 sigma bond and 1 pi bond. Since the pi bond is formed by lateral overlap and is weaker than the sigma bond formed by linear overlap, the pi bond is easily broken in the reactions of ethylene.
- Alkenes : Aliphatic Unsaturated Hydrocarbons
- How is a chemical equation written in chemistry?
- 14 Most Important Reactions in Chemistry
- The Gas Laws: Charle’s Law, Boyle’s Law and Gay-Lussac's law
- Hydrogen Bond : Sigma bond and Pi bond with example
Like ethylene in other alkenes also 1 pi bond is present. Due to the presence of pi bond, these compounds are highly reactive and show additive reactions. For this reason these compounds are called unsaturated compounds.
The bond angle in sp2 hybridization is 120°. Therefore, the value of each H–C–H and H–C–C angle in ethylene molecule is 120° and ethylene molecule is planer. In alkenes, the C–C bond length is 1.34 A and the C–H bond length is 1.60 A. The C = C binding energy is 145 k cal per mole.
General Methods of Preparation of Alkenes
By Dehydration of Alcohols : Alkene is obtained by heating alcohols with concentrated sulfuric acid or concentrated phosphoric acid.
Example-
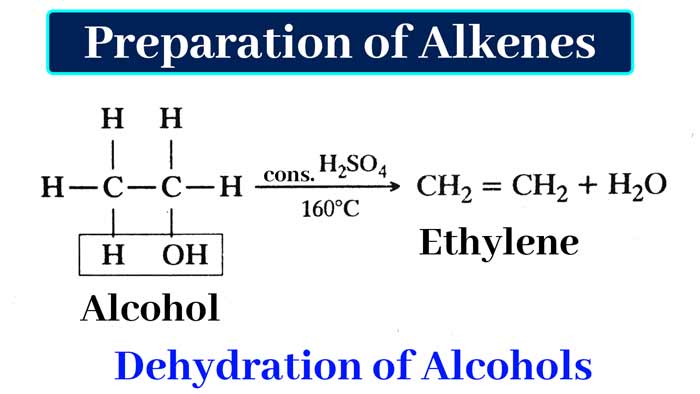
In this reaction one molecule of water is removed from one molecule of alcohol. Such reactions are called dehydration.
Dehydration of alcohols can also be done by passing its vapors over heated alumina (Al2O3) or anhydrous aluminum sulfate.
In this reaction one molecule of water is removed from one molecule of alcohol. Such reactions are called dehydration.
Dehydration of alcohols can also be done by passing its vapors over heated alumina (Al2O3) or anhydrous aluminum sulfate.
CH3 – CH2OH → CH2 = CH2 + H2O
The order of ease of dehydration in different alcohols is as follows –
Tertiary Alcohol > Secondary Alcohol > Primary Alcohol
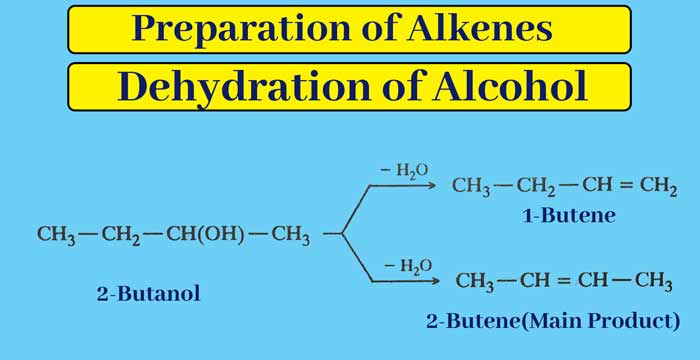
If the dehydration of an alcohol can yield more than one alkene, then dehydration occurs according to Saytzeff’s rule. In the dehydration of alcohols, the water molecule is obtained by the separation of the OH group present on one carbon atom and the hydrogen atom present on another adjacent carbon atom.
According to Saytzeff’s rule, hydrogen is obtained from the nearest carbon atom which has less number of hydrogen atoms already present.
Example: The main product of dehydration of 2-butanol is 2-butene.
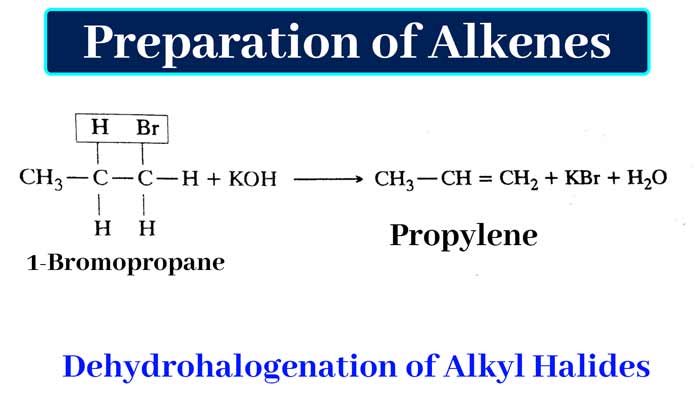
By dehydrohalogenation of alkyl halides: Alkene is obtained by heating alkyl halides with an alcoholic solution of caustic potash. In this process one molecule of hydrogen halide is removed from one molecule of alkyl halide. This irreversible process is called dehydrohalogenation.
Example :
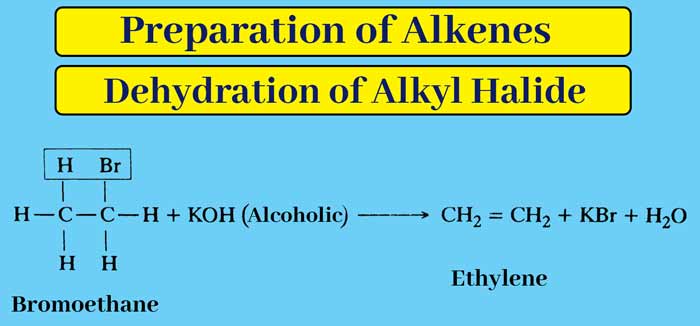
If the dehydrohalogenation of an alkyl halide can yield more than one alkene, the reaction follows Saytzeff’s rule.
By dehalogenation of Dihalo alkanes :- Dihalo alkanes in which two halogen atoms are present on two adjacent carbon atoms are called vicinal dehalides.
On heating such dihalos with zinc powder, alkene is obtained.
Example :-

By electrolysis of dicarboxylic acids :-
Kolbes reaction :- Alkenes are obtained at the anode by electrolysis of aqueous solutions of sodium or potassium salts of dicarboxylic acids.
Example: Electrolysis of aqueous solution of potassium succinate gives ethylene at the anode.

By partial reduction of alkynes: The end product of the addition reaction of alkynes with hydrogen is alkane. In this reaction, Ni is used as a catalyst and the temperature is kept at 250 – 300°C. If alkynes are taken in large quantity and the reaction is carried out at low temperature, then alkene is also obtained as a result of the reaction.
example:-
CH≡CH + H2 → CH2 = CH2
In the above reaction there is partial hydrogenation of acetylene i.e. partial reduction. In this reaction, out of the two pi bonds present in acetylene, one pi bond is broken and one pi bond remains unaffected. Partial hydrogenation of alkynes can also be done by hydrogen in the presence of Lindlar catalyst or by dissolution of sodium in liquid ammonia.
Example:-
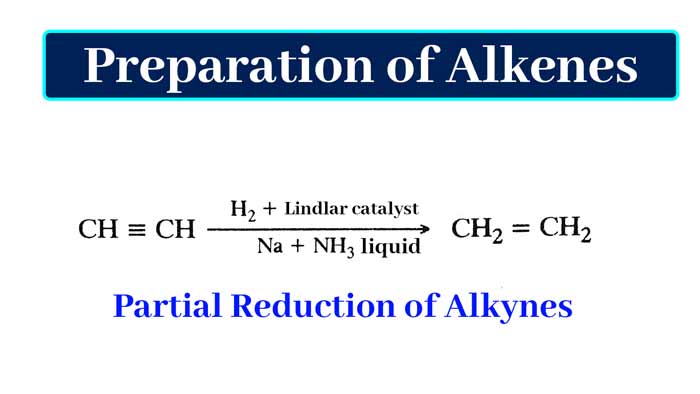
As a result of partial hydrogenation by hydrogen in the presence of Lindlar catalyst, the neutral alkene is obtained as a result of partial hydrogenation of sodium alkene and sodium in liquid ammonia.
example-
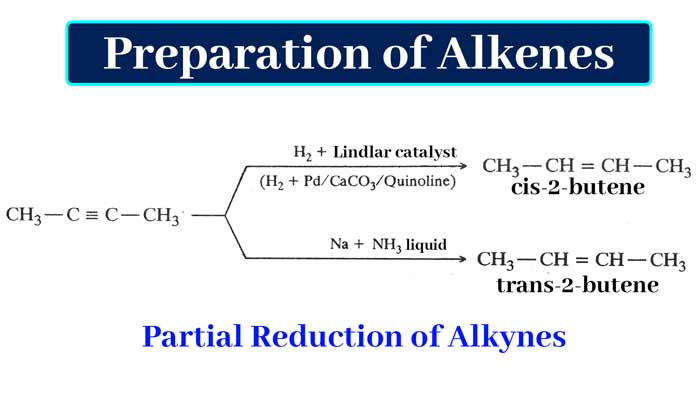
The mixture of palladium and quinoline containing calcium carbonate is called Lindlar catalyst. In this mixture Pd/CaCO3 acts as catalyst and quinoline as catalyst poison. In this mixture, in place of CaCO3, BaSO4 and lead acetate can also be taken in place of Quinoline.
By Cracking of Alkanes: On heating alkanes in the presence of air at 500-700°C temperature, their molecules split into molecules of less mass. The mixture obtained contains the following alkane, alkene and hydrogen.
example:
2CH3 – CH2 – CH3 → CH3 – CH = CH2 + CH2 = CH2 + CH4 + H2
The components of the obtained mixture can be separated by suitable methods.
Grignard Reagents:- Higher alkenes can be obtained by the reaction of halogen substituted alkenes and gridnard reagents.
example :
CH2 = CH – CH2Cl + CH3 – Mg – Cl → CH2 = CH – CH2 – CH3 + MgCl2
Tetra alkene derivatives of ammonium hydroxide : On heating tetra alkene derivatives of ammonium hydroxide give an alkene.
example :
(C2H5)4N – OH → C2H4 + (C2H5)3N + H2O
[(C2H5)4N – OH = Tetraethylammonium Hydroxide]
[(C2H5)3N = Triethylamine]