Hydrogen Bond : Sigma bond and Pi bond with example
Hydrogen Bond
The bond which is formed between an atom of hydrogen and an atom of a negative electronegative element due to the mutually stable electrostatic attraction force of their partial positive and negative charges, is called hydrogen bond.
A covalent bond between a hydrogen and an atom of a strongly electronegative element is a polar covalent bond. For this reason, there is a partial positive charge on the hydrogen atom and a partial negative charge on the atom of the negative electronegative element.
Example: In Hydrogen Fluoride (HF), the bond formed between H and F is a polar covalent bond and due to this there is a partial positive charge on H and a partial negative charge on F.
H+ – F–
That atom of hydrogen which is connected to an atom of a negative electronegative element by covalent bond, can also be attached to any other atom of that negatively electronegative element by means of a stable electrostatic attraction force. This type of bond is called hydrogen bond and it is represented by dotted lines ( – – -).
In this case, the atomic negative electronegativity of Hydrogen forms a bridge between the two atoms of the element. The strength of hydrogen bond depends on the negative electronegativity of the negative electronegativity element.
When the negative power is high, the strength is high. The following is the sequence of occurrence of negative energies of the major elements.
F > O > Cl ~ N > Br > C ~ S ~ I > H
If the atoms of hydrogen and negative electronegativity between which hydrogen bond is formed are part of different molecules of a compound, then this type of bond is called intermolecular hydrogen bonding.
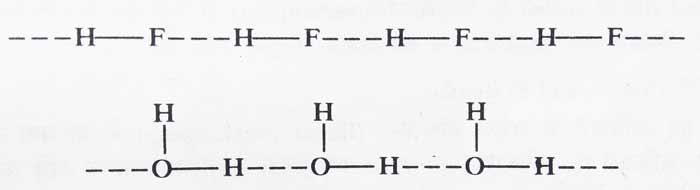
Example: Intermolecular hydrogen bonding occurs in HF and H2O.
Compounds which have intermolecular hydrogen bonding, their molecules are attracted to each other due to hydrogen bonding. Therefore, more energy is required to separate the molecules of these compounds. Therefore, the melting and boiling points of such compounds are often high.
Example: HF and H2O are liquids at room temperature and NH3, HCl and H2S are gases.
- Electric Potential Energy : Electric Dipole, Potential Gradient
- Coulomb's Law : Electric Field, Potential Difference, Volt
- Gauss Law or Gauss Theorem : Solid Angle and Electric Flux
- Electrolysis : Definition, Principle and Electrolytic Cell
- Adsorption : Adsorption rate, Physisorption and Chemisorption
- Electric Cell : E.M.F., Terminal Potential, Internal Resistance
In other words, the boiling points of HF and H2O are higher than those of NH3, HCl and H2S. The reason for this is that F and O are strongly negatively electronegative elements, so hydrogen bonding is present in HF and H2O.
In HF and H2O there are hydrogen bonds between atoms of different molecules. Therefore, intermolecular hydrogen bonding takes place in HF and H2O. N , Cl and S are not so strongly negatively electronegative elements and hydrogen bonding in NH3, HCl and H2S is not very or very weak.
Therefore, the boiling points of HF and H2O are higher than those of HCl, H2S and NH3. Compounds which have intermolecular hydrogen bonding, when water is added to them, the molecules of the compound form hydrogen bonds with water molecules. Therefore, these types of compounds are usually soluble in water.
If the atoms of hydrogen and the negative electronegative element, between which hydrogen bond is formed, are part of a molecule of a compound, then this type of bonding is called intramolecular hydrogen bonding.
Example: Intramolecular hydrogen bonding occurs in ortho nitrophenol. Similarly, intramolecular hydrogen bonding occurs in selycylaldehyde and celycilic acid.
Compounds that have intramolecular hydrogen bonding usually have low melting and boiling points and are volatile.
Example: Intermolecular in aortho nitrophenol and intramolecular hydrogen bonding in per nitrophenol. The melting point of aortho nitrophenol is 45°C while the melting point of pera nitrophenol is 114°C. Aortho nitrophenol steam is volatile. Whereas pera nitrophenol is not water volatile.
Compounds that have entermolecular hydrogen bonding are often insoluble in water or have low solubility in water.
Example: The solubility of aurtho nitrophenol in water is less than that of pera nitrophenol in water.
Concentration of the Ore
According to the modern concept of atomic structure, electrons in an atom reside in different orbitals. There can be a maximum of two electrons in any one orbital and their spin is in the opposite direction.
If the second electron is acquired by transfer or sharing by the orbitals which have only one electron, then the direction of spin of this second electron is opposite to the direction of spin of the first electron.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
The opposite direction of the magnetic field produced by the spins of both electrons results in neutralization of the magnetic field and loss of energy, resulting in stability.
Based on the knowledge of atomic structure, it is necessary to explain how the orbitals participate in the sharing of electrons when covalent bonds are formed by the sharing of electrons. Two theories have been proposed to explain the sharing of electrons with respect to orbitals.
- valency bond theory
- molecular orbital theory
According to both these theories, those orbitals of the atom which are half-filled i.e. which have only one electron, participate in forming covalent bonds. Covalent bonds are formed by overlapping of half filled orbitals. This theory of formation of covalent bond by overlapping of orbitals is called orbital theory of covalency.
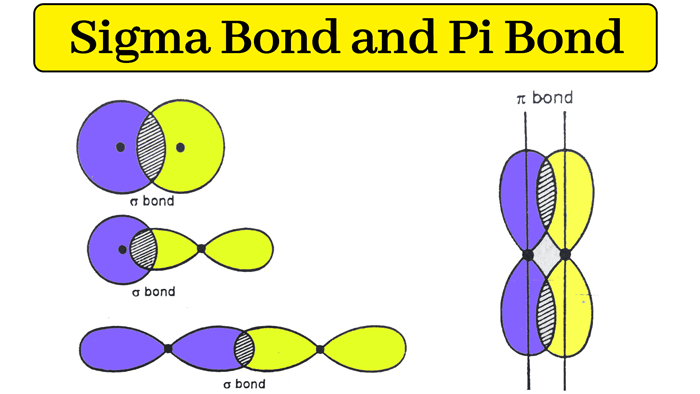
Sigma and Pi Bonds
The bond formed by the linear overlapping of two half-filled orbitals is called a Sigma bond(σ – Bond).
When a sigma bond is formed by the overlapping of two orbitals, the axes of these orbitals are in the same straight line while overlapping.
The bond formed by the sidewise overlapping of two half-filled orbitals is called a Pi bond. When pi bond is formed by overlapping of two orbitals, the axes of these orbitals are parallel to each other while overlapping.
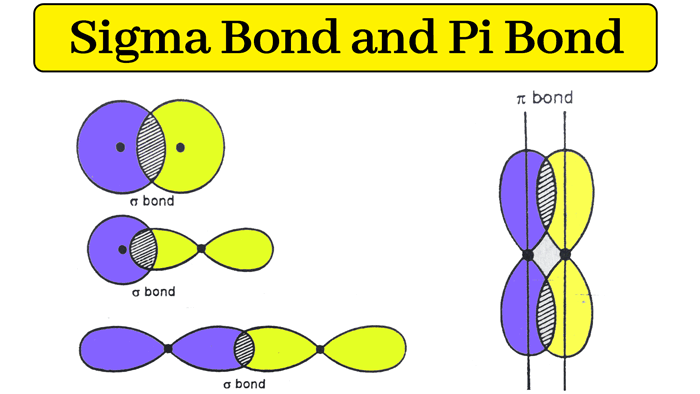
Example: hydrogen atom (H2) : The electronic configuration of hydrogen atom (H) is 1s1.
When two hydrogen atoms combine to form a hydrogen atom, the overlapping of the s orbitals of these atoms forms a sigma bong.
Therefore, the single bond in a hydrogen atom is a sigma bond.
Oxygen atom: The electronic configuration of oxygen (O) is 1s2, 2s2, 2px2, 2py1, 2pz1. It has two half-filled p orbital in its valence shell. When two oxygen atoms combine to form an oxygen molecule, a p orbital of one atom is linearly overlapping with a p orbital of another atom to form a sigma bond.
The remaining p orbitals on each atom are parallel to each other. Their lateral overlap forms a pi bond. Therefore, in the double bond located in the oxygen atom, there is a sigma and a pi bond.
Nitrogen Atom: The electronic configuration of nitrogen (N) is 1s2, 2s2, 2px1, 2py1, 2pz1. It has three half-filled p orbital in its valence shell. When two nitrogen atoms combine, a sigma and 2 pi bonds are formed between them.
Hydrogen Chloride: In HCl, a sigma bond is formed between H and Cl by overlapping between the s orbital of H and the p orbital of Cl.
Strength of sigma and pi-bonds
According to the orbital theory of co-valency, the bonds formed by lateral overlapping are weaker than the bonds formed by linear overlapping. So sigma bond is strong bond and pi bond is weak bond. sigma bonds are less reactive and pi bonds are more reactive. The stability of sigma bond is high and the stability of pi bond is low.
Characteristics of single, double and tri bonds
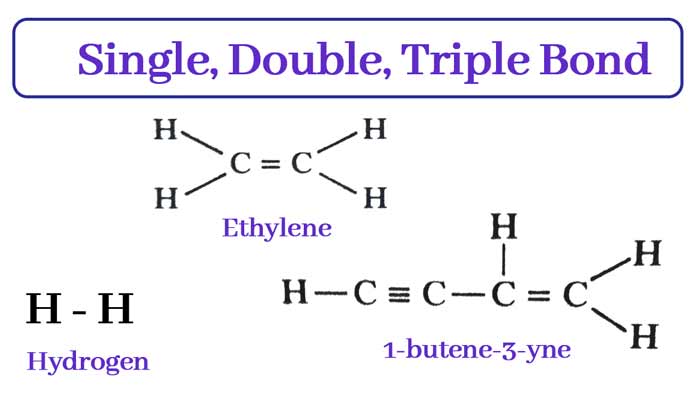
Observing the overlapping of orbitals in different molecules leads to the following conclusions.
- Single bond = One sigma
- Double bond = one sigma + one pi bond
- Triple bond = one sigma + two pi bond
Therefore, from the structure formula of any compound, the sigma and pi bonds present in it can be calculated.
Example:
A molecule of ethylene (C2H4) has four single bonds and one double bond. Therefore, one molecule of ethylene has 5 sigma and 1 pi bond.
1 butyne 3 ion (C4H4) has 5 single bonds, one double bond and one triple bond. Therefore, a molecule of 1 butene 3 yne has 7 sigma bonds and 3 pi bonds.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
Bond Energy: When a triple bond breaks completely, one sigma and two pi bonds break. When a double bond breaks completely, a sigma and a pi bond are broken. When a single bond breaks, only one sigma bond is broken.
Therefore, the maximum energy will be required to break the triple bond and the least energy will be required to break the single bond. This conclusion has been confirmed by experiments.
The binding energies of C ≡ C , C = C and C – C have been found to be 839, 614 and 347 kJ/mole respectively. Hence, the increasing order of binding energy of single double triple bond is as follows –
Increasing order of binding energy: Single bond < Double bond < Triple bond
Bond Strength: Bond energy is the measure of bond strength. The higher the bond energy, the higher will be the bond strength. Therefore, the increasing order of strength of single, double and triple bonds is as follows –
Increasing order of bond strength : single bond < double bond < triple bond
Activity of Bonds: Due to the presence of two pi bonds, triple bonds are easily converted into double bonds or single bonds. Similarly, due to the presence of a pi bond, double bonds are easily converted into single bonds. Therefore, double and triple bonds are more reactive than single bonds.
There are one pi bond in double bond and two pi bond in double bond. Pi bonds are weak bonds and due to this they are more reactive. Hence it can be inferred that the triple bond will be more reactive than the double bond.
Therefore, the reactivity of acetylene is likely to be higher than that of ethylene. In fact, ethylene is more reactive than acetylene in addition reactions. Therefore, in this case, it is more active than the double bond.
Stability of Bonds: Double bond and triple bond are more reactive than single bond. Therefore, the stability of a single bond is higher than that of double and triple bonds.
Bond Length: The bond length of carbond single bond, carbon double bond, and carbon triple bond is as follows –

It is clear that the decreasing order of bond length is –
C – C > C = C > C ≡ C
The decreasing order of bond length of single, double and triple bonds among other atoms is also the same.