Chlorine Gas – Laboratory and Industrial Preparation
Chlorine Gas History
Chlorine gas was discovered by Scheele in 1774. Carl Wilhelm Scheele found that on heating Manganese dioxide with hydrochloric acid, a greenish-yellow gas is obtained. Initially it was assumed that it is a compound. Davy proved in 1810 that it is an element and named it Chlorine (Greek: Chloros = greenish yellow).
Chlorine Gas Presence
Being more active, it is not found in free state in nature. It is found in combined state as chloride salts.
Example: Sodium chloride (NaCl), potassium chloride (KCl), and magnesium chloride (MgCl2) are found in abundance in sea and rocks.
Common Methods for Making Chlorine Gas
Common Methods for Making Chlorine Gas
Chlorine gas is obtained when hydrochloric acid is oxidized by any oxidizing substance.
Example :
From Manganese dioxide MnO2 :
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
From Potassium permanganate KMnO4 :
2KMnO4 + 16HCl → 2MnCl2 + 2KCl + 8H2O + 5Cl2
From Potassium dichromate K2Cr2O7 :
K2Cr2O7 + 14HCl → 2CrCl3 + 2KCl + 7H2O + 3Cl2
From Potassium dichromate K2Cr2O7 :
By chlorides of metals
When chloride of any metal is heated with MnO2 and concentrated H2SO4, chlorine gas is obtained.
Example: With NaCl –
2NaCl + 2H2SO4 → 2NaHSO4 + 2HCl
MnO2 + H2SO4 → MnSO4 + H2O + O
2HCl + O → H2O + Cl2
X ===== X ===== X ===== X ===== X ===== X
2NaCl + MnO2 + 3H2SO4 → 2NaHSO4 + MnSO4 + 2H2O + Cl2
By the action of acids on Hypochlorites
Chlorine gas is also obtained by the action of HCl or H2SO4 on sodium hypochlorite (NaOCl) or bleaching powder caoCl2.
NaOCl + 2HCl → NaCl + H2O + Cl2
CaOCl2 + H2SO4 → CaSO4 + H2O + Cl2
Laboratory Methods for Preparation of Chlorine
By heating MnO2 and concentrated HCl
Taking Magnese dioxide(MnO2) in a flask, concentrated hydrochloric acid is poured into it with the help of thistle funnel. This device is air tight. When the mixture is heated, chlorine gas is released.
- Electrochemistry : Electrolytic cell, Mechanism and Cell Structure
- Dalton's law of partial pressure || Numeric Example
- Hydrogen : Properties, Preparation, Purification and uses
- NEET Thermodynamics Questions : NEET Previous Year questions
- NEET Solid State Questions : NEET Previous Year Questions
It is collected by upward displacement of air in a gas jar after passing it alternately in water and concentrated H2SO4 respectively.
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
By reaction of concentrated HCl on KMnO4
This is the simplest method to prepare chlorine gas in the laboratory. In this method, potassium permagnate(KMnO4) is taken in a flask and concentrated hydrochloric acid is added drop by drop with the help of thistle funnel.
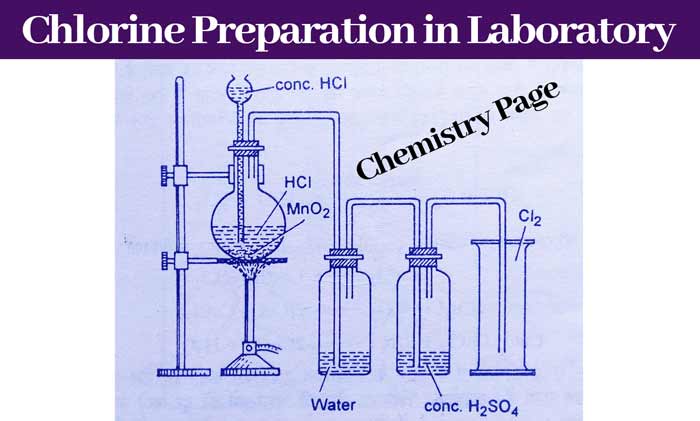
By doing this, chlorine gas starts forming rapidly, which is first passed into water and then passed into concentrated sulfuric acid and collected in a gas jar by upward displacement of air.
2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2
By the reaction of concentrated HCl on K2Cr2O7:
In the laboratory chlorine gas is also prepared by heating potassium dichromate and concentrated dichloric acid.
K2Cr2O7 + 14HCl → 2KCl + 2CrCl3 + 7H2O + 3Cl2
Industrial manufacture of Chlorine Gas
There are two main methods of industrial manufacture of Chlorine – Deacon method and Electrolytic method.
Deacon’s method:
In this method, chlorine gas is made by oxidizing hydrogen chloride gas by oxygen in the air in the presence of a catalyst. In this process, cupric chloride (CuCl2) is taken as a catalyst.
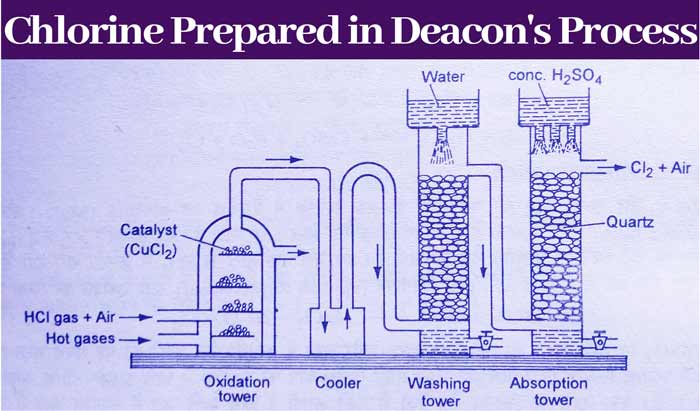
In this method a plant is prepared. First of all, hydrogen chloride gas is formed by the action of concentrated sulfuric acid on sodium chloride (Salt NaCl) and it is mixed with air and sent to an oxidising column.
The temperature of this column is 450°C and cupric chloride is kept in it as a catalyst. In this column, hydrogen chloride is oxidized by the oxygen of the air and chlorine gas is formed.
4HCl + O2 → 2H2O + 2Cl2
The catalytic reactions of cupric chloride are represented by the following equations:
2CuCl2 → Cu2Cl2 + Cl2
2Cu2Cl2 + O2 → 2[CuO . CuCl2]
CuO . CuCl2 + 2HCl → 2CuCl2 + H2O
Along with chlorine, hydrogen chloride gas, water vapor and remaining air are also present in the form of impurities in the gases obtained from the oxidizing column.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
To remove the impurities of hydrogen chloride gas, this gaseous mixture is cooled and sent to the washing tower first. In this tower, water is rained from above, due to which hydrogen chloride gas dissolves in water to form hydrochloric acid.
In this way the impurity of HCl gas gets removed in this tower. The gaseous mixture obtained from this tower is sent to the drying tower, in which drops of concentrated sulfuric acid continue to drip from the top.
The water vapor is absorbed by concentrated sulfuric acid and separated from the gaseous mixture. The gases received from this tower mainly consist of chlorine gas, but the impurities of the air also remain.
Electrolytic method
This is a modern method of manufacturing chlorine gas. In this method, electrolysis of aqueous sodium chloride solution (Brine) or molten sodium chloride is done. The following is a description of the cells used for the electrolysis of sodium chloride.
Nelson cell
This cell consists of a U shaped steel perforated tube which is used as a cathod. In this cell, a rod of graphite acts as anode. In this cell between the anode and the cathode, an aqueous solution of sodium chloride is filled, which acts as an electrolyte.
A porous diaphragm of asbestos is placed between the electrolysis and the cathode. The following actions take place when current is passed through the electrodes –
NaCl ⇌ Na+ + Cl–
2H2O + 2e– → H2(g) + 2OH– (Cathode)
Na+ + OH– ⇌ NaOH
2Cl– → Cl2(g) + 2e– (Anode)
The complete and balanced equation of the reaction is as follows –
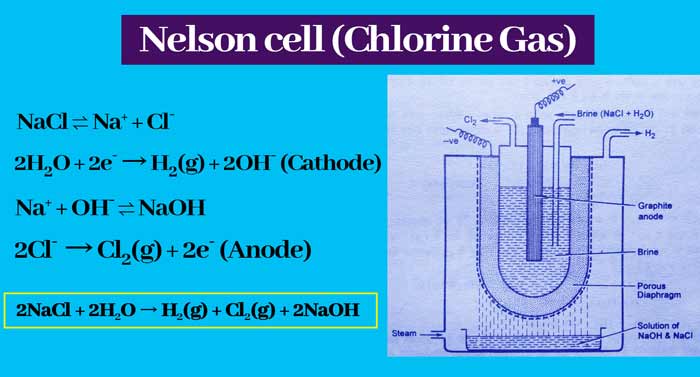
2NaCl + 2H2O → H2(g) + Cl2(g) + 2NaOH
Thus chlorine gas is obtained at the anode.
Castner Kellner Cell
Like the Nelson cell, this cell is also used for the industrial manufacture of chlorine by the electrolyte of an aqueous solution of sodium chloride.
It is in the shape of a rectangular vessel and has three parts. A dilute aqueous solution of sodium hydroxide is filled in the middle part and concentrated aqueous solution of sodium chloride (Brine) is filled in both the outer parts.
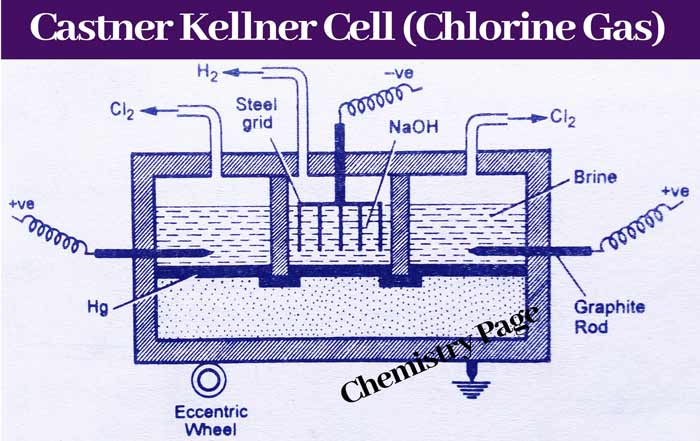
In the middle part a cathode made of iron strips is inserted and in the outer part the graphite rods are immersed in the solution, which acts as an anode.
A layer of mercury is made on the bottom of the vessel, which remains in contact with the three parts of the vessel in such a way that the solutions filled in these parts do not come in contact with each other.
The mercury layer can be rotated with the help of an eccentric wheel so that the Na+ formed in the left and right chambers can be brought to the middle chamber from time to time with the help of the murcury layer.
Due to the ionization of sodium chloride in the outer parts of the pot, sodium ion (Na+) and chloride ion (Cl–) are present. Chlorine gas is created by going to the chloride ion anode when electricity is passed between the electrodes.
NaCl ⇌ Na+ + Cl–
2Cl– → Cl2(g) + 2e– Graphite (Anode)
Na+ + Hg → (Na . Hg)+
Hydrogen gas and hydroxide ions are obtained as a result of reduction of H2O in the middle of the vessel. Thus, there is an excess of Na+ in the outer part and OH– in the middle part. The sodium ions reach the surface of mercury as well as in the middle part where they combine with hydroxide ions to form sodium hydroxide.
2H2O + 2e– → H2 + 2OH– Steel (Cathode)
(Na . Hg)+ → Na+ + Hg
Na+ + OH– ⇌ NaOH
Thus the concentration of sodium hydroxide in the middle part keeps on increasing. The concentrated solution of sodium hydroxide is taken out in due time. Thus this cell is also used for industrial manufacture of sodium hydroxide.
Solvays trough cell
This cell is a modified form of Castner cell. In this, the rod of the graphite acts as the anode and about 1/3 of the thickness of the murcury surface acts as the cathode.
During electrolyte, the surface of mercury keeps flowing slowly from one side to the other at the bottom of the vessel.
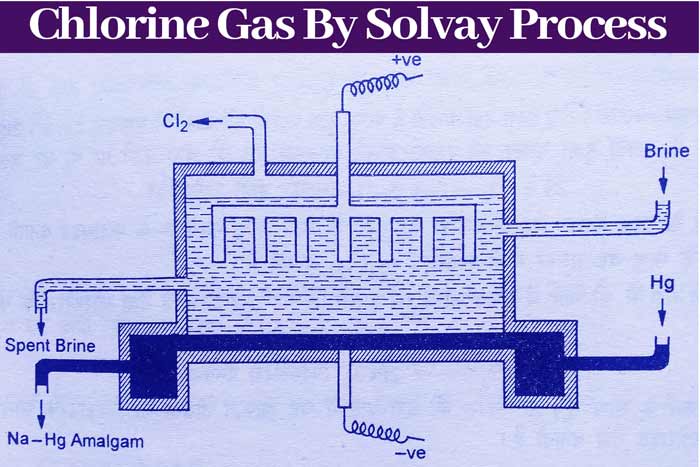
The solution of sodium chloride is kept in the cell from one side and the used solution is removed from the other side in such a way that the direction of flow of mercury and sodium chloride in the cell is same. The solution method to the droni cell is shown below.
Reaction:
As a result of ionization of sodium chloride, sodium and chloride ion are obtained –
NaCl ⇌ Na+ + Cl–
Chlorine gas is made by going to the chloride ion in anode –
2Cl– – 2e– → Cl2 (Graphite in Anode)
By going to sodium ion in cathode, make amalgam with mercury.
Na+ + Hg → (Na . Hg)+
(Na . Hg)+ + e → Na . Hg (Mercury in Cathode)
When sodium mercury amalgam obtained from the cell is reacted with water, sodium hydroxide is obtained –
Na . Hg → Na + Hg
2Na + 2H2O → 2NaOH + H2
When sodium mercury amalgam obtained from the cell is reacted with water, sodium hydroxide is obtained –
Down Cell
This cell is used for industrial manufacture of chlorine gas by electrolyte of molten sodium chloride. The anode in this cell is made of graphite and made of steel. On passing electricity between the electrodes, sodium chloride becomes an electrolyte and chlorine gas is obtained at the anode.
NaCl ⇌ Na+ + Cl–
2 Cl– → 2e– + Cl2 (Anode)
Na+ + e– → Na (Cathode)
