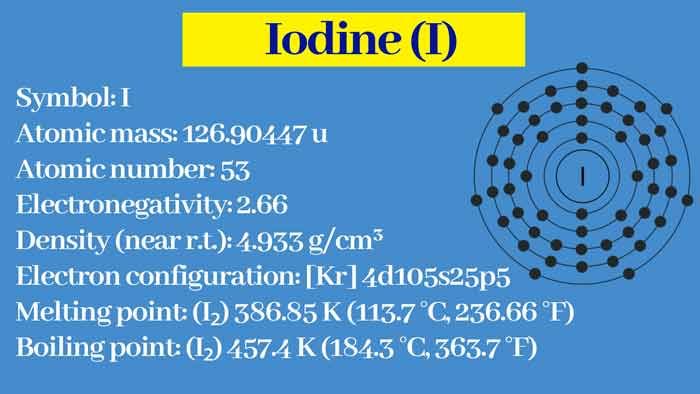
Iodine : Properties, Preparation and uses
Iodine History
Iodine was discovered by Bernard Courtois in 1811. Its vapors are purple in colour. For this reason Galusek named it Iodine (Greek: Iodas = Violet) in 1813. Humphry Davy proved in 1813 that it is an element.
Iodine Presence
Iodine is not found in free state in nature. In the combined state, it is found in the form of iodides. Chile saltpeter mainly contains NaNO3 and it is also called caliche. In Chile saltpeter it is found as sodium iodate and in sea weeds it is found as iodides of sodium and potessium. Sea grass and tree plants are called sea weeds.
Iodine Preparation
Common Methods for Making Iodine
Iodine is formed by adding bromine liquid to an aqueous solution of potassium iodide or passing chlorine gas.
2KI + Br2 → 2KBr + I2
2KI + Cl2 → 2KCl + I2
Adding potassium iodide to copper sulfate solution also forms iodine.
2CuSO4 + 4KI → Cu2I2 + 2K2SO4 + I2
Iodine is formed on heating a mixture of potassium iodide, magnesium dioxide and concentrated sulfuric acid.
2KI + MnO2 + 3H2SO4 → 2KHSO4 + MnSO4 + 2H2O + I2
Laboratory Methods for Preparation of Iodine
Iodine is prepared in the laboratory by heating a mixture of KI, MnO2 and concentrated H2SO4. Idonine is formed by heating this mixture.
Iodine is a solid but it readily converts into vapor state on heating. The vapors of iodine obtained from the above reaction are condensed at the bottom of an ice-cold cup to obtain iodine as a solid.

Oxidizers like Ozone, Hydrogen Peroxide, Acidic Potassium permagnate, acidic potassium dicromate can also oxidise potassium iodide to iodine.
Example:
2KI + H2O2 → 2KOH + I2
By the reaction of sodium hydrogen sulfite on sodium iodate –
2NaIO3 + 5NaHSO3 → 3NaHSO4 + 2Na2SO4 + H2O + I2
By the reaction of dilute sulfuric acid on a mixture of potassium iodate and potassium iodide
KIO3 + 5KI + 6H2SO4 → 6KHSO4 + 3H2O + 3I2
By the reaction of dilute sulfuric acid on a mixture of potassium iodate and potassium iodide
Industrial Methods of Making Iodine
Sea weeds: Sea grasses and sea plants are called sea weeds. Of these, the amount of iodine is high in the stems of Laminaria digitata and Laminaria stenophylla. The amount of iodine is also high in weeds obtained from the deepest depths of the sea.
Sea weed is dried and burnt. The ashes obtained by burning are called Kelp. Lixivate the mixture a lot by mixing kelp with water. By doing this iodide, bromide, sulfate, carbonate, chloride, and some other salts of sodium and potassium become soluble in water.
- Iodine : Properties, Preparation and uses
- Bromine – Preparation, Physical and Chemical Properties a Uses
- Chlorine Property : Physical and Chemical Properties | Uses
- Solar Energy : Light Waves, Reactions and Uses
- Chlorine Gas – Laboratory and Industrial Preparation
On filtering and concentrating the above mixture, sodium and potassium are separated in the form of sulfate, carbonate and chloride crystals. The filter solution obtained is called mother liquor. In this, mainly iodide of potassium and sodium are present in aqueous solution.
2NaI + MnO2 + 3H2SO4 → 2NaHSO4 + 2H2O + MnSO4 + I2
2NaI + MnO2 + 3H2SO4 → 2KHSO4 + 2H2O + MnSO4 + I2
Small amounts of sodium and potassium bromide are also present in it. Concentrated sulfuric acid is mixed in it and kept for some time, due to which the sulfur gets precipitated. MnO2 is added to the sifted liquid and heated in retart.

The impurities of the bromides of sodium and potassium also react with MnO2 and H2SO4 and form bromine.
2NaBr + MnO2 + 3H2SO4 → 2NaHSO4 + 2H2O + MnSO4 + Br2
2KBr + MnO2 + 3H2SO4 → 2KHSO4 + 2H2O + MnSO4 + Br2
The vapors of iodine obtained from Retort are cooled in aludels. Iodine is deposited as a solid on the walls of the aludels. It is separated by scraping and by sublimation it is obtained in pure state.
Chile saltpeter (Caliche): – Chile saltpeter mainly contains sodium nitrate but it also contains sodium iodate (NaIO3) in some amount. After dissolving caliche in water and concentrating the aqueous solution, most of the sodium nitrate gets separated in the form of crystals.
2NaIO3 + 5NaHSO3 → 2NaHSO4 + 2Na2SO4 + H2O + I2
A solution of sodium bisulfite is added to the obtained solution, due to which a blue-violet precipitate of iodine is obtained.
- Iodine : Properties, Preparation and uses
- Bromine – Preparation, Physical and Chemical Properties a Uses
- Chlorine Property : Physical and Chemical Properties | Uses
- Solar Energy : Light Waves, Reactions and Uses
- Chlorine Gas – Laboratory and Industrial Preparation
Filter this precipitate and wash it with water and dry it. This is the main method of making iodine on an industrial scale.
Refinement of Industrial Iodine
In the sample of iodine obtained by the above methods, the impurities of chlorine and bromine are found in the form of iodine chloride and iodine bromide.
ICl + KI → KCl + I2
IBr + KI → KBr + I2
These impurities cannot be removed even after repeating the process of sublimation. To remove these impurities, an impure sample of iodine is mixed with potassium iodide and sublimated. In this way pure iodine is obtained by sublimation.
Physical Properties
It is a dark blue colored solid material. It is a crystal and also volatile. Its melting point is 114°C and boiling point is 185°C. It can be sublimated. That is, on rapid heating, it directly changes into the gas state and on rapid cooling of its vapors, it directly changes into the solid state.
It is soluble in small amounts in water. It is soluble in organic solvents such as: chloroform, carbon tetra chlodie, benzene and carbon di sulfide. It is also soluble in aqueous solution of potassium iodide.
KI + I2 ⇌ KI3
Aqueous solutions of KI and I2 have a dark yellow color due to the formation of KI3.
Chemical Properties
Combination with elements:
Iodine directly combines with many elements to form iodide compounds.
Example :
Combination with hydrogen : In normal state it does not react with hydrogen but on heating in the presence of catalyst (Pt) it combines with hydrogen.
H2 + I2 → 2HI
Combination with other halogens: It combines with other halogens to form interhalogen compounds.
Example:
When chlorine is passed over solid iodine at a temperature below 0°C, iodine monochloride ICl is formed.
I2 + Cl2 → 2ICl
Iodine trichloride is formed by the reaction of excess of iodine and chlorine at 100°C temperature.
I2 + 3Cl2 → 2ICl3
Combination with other elements:
Some other examples of combination with some other elements under suitable conditions are as follows –
2P + 3I2 → 2PI3 (Phosphorus triiodide)
Hg + I2 → HgI2 (Mercuric iodide)
2K + I2 → 2KI (Potassium iodide)
2Sb + 3SbI3 (Antmani tri-iodide)
Reaction with sodium hydroxide:
Its reaction with sodium hydroxide is similar to that of chlorine and bromine. It reacts with cold and dilute NaOH to form sodium hypo iodite and sodium iodide.
2NaOH + I2 → NaI + NaOI + H2O
With hot and concentrated NaOH it forms sodium iodide and sodium iodate.
6NaOH + 3I2 → NaIO3 + 5NaI + 3H2O
Reaction with Ammonia:
Its reaction with ammonia is similar to that of chlorine and bromine. If ammonia content is high then the following reaction takes place.
8NH3 + 3I2 → 6NH4I + N2
If the amount of iodine is high, the explosive substance nitrogen tri iodide (NI3) is formed.
3I2 + NH3 → NI3 + 3HI
- Iodine : Properties, Preparation and uses
- Bromine – Preparation, Physical and Chemical Properties a Uses
- Chlorine Property : Physical and Chemical Properties | Uses
- Solar Energy : Light Waves, Reactions and Uses
- Chlorine Gas – Laboratory and Industrial Preparation
- Sulfuric Acid : Chemical Properties, Uses and Structure
- Rigid Body: Moment of Force or Torque formula
- Preparation of Sulphuric Acid by Contact process with Reaction
Oxidative property:
It can act as an oxidising agent. Its main oxidising reactions are as follows –
Oxidizer of SO2: It oxidises SO2 to H2SO4.
I2 + SO2 + 2H2O → H2SO4 + 2HI
Oxidation of H2S: It oxidises H2S to S.
I2 + H2S → 2HI + S
Oxidation of Sulfites: It oxidises sulfites to sulfates.
example :
Na2SO3 + H2O + I2 → Na2SO4 + 2HI
Oxidation of nitric oxide: It oxidises NO to HNO3.
2NO + 4H2O + 3I2 → 2HNO3 + 6HI
Oxidation of stannous chloride: It oxidises SnCl2 to SnCl4.
SnCl2 + 2HCl + I2 → SnCl4 + 2HI
Oxidation of hypo: It oxidises sodium thiosulfate (Hypo) to sodium tetrathionate. It is an important reaction of iodine and is used in quantitative analyses.
2Na2S2O3 + I2 → Na2S4O6 + 2NaI
Oxidation of hypo: It oxidises sodium thiosulfate (Hypo) to sodium tetrathionate. It is an important reaction of iodine and is used in quantitative analyses.
Reducing properties:
It reacts with various oxidising agents to form iodic acid. It acts as a reducing agent in these reactions.
Example:
Reaction with chlorine water:
I2 + 5Cl2 + 6H2O → 2HIO3 + 10HCl
Reduction of ozone:
I2 + 5O3 + H2O → 2HIO3 + 5O2
Reduction of nitric acid:
3I2 + 10HNO3 → 6HNO3 + 10NO + 2H2O
It reacts with a mixture of sodium sulfide and sodium sulfite to form sodium thiosulfate (Hypo). In this reaction, iodine is taken in small quantity, otherwise it reacts with the product (sodium thiosulfate) to form another product (sodium tetrathionate).
Na2S + Na2SO3 + I2 → Na2S2O3 + 2NaI
Reaction with KClO3 : In the presence of HNO3 it converts potassium chlorate (KClO3) to potassium iodate (KIO3).
2KClO3 + I2 → 2KIO3 + Cl2
Action with ethyl alcohol: reacts with ethyl alcohol in the presence of alkali to form iodoform
C2H5OH + 6NaOH + 4I2 → CHI3 + HCOONa + 5NaI + 5H2O
Reaction with starch: It gives dark blue color due to formation of starch iodide salt hybrid compound with starch solution. The blue color disappears on heating but it regains on cooling. This reaction is used to test iodine.
Iodine Uses
Iodine and some of its compounds are used to make medicines.
example :
Tincture of iodine is obtained by dissolving I2 in aqueous solution of KI and adding rectifide strite (95% ethyl alcohol) to it. Due to the yellowish brown color of KI3, the color of tincture iodine also remains yellowish brown. This solution is used as a bactericidal to clean wounds.
Iodoform is used as a bactericide.
Iodine is also used to make iodex. iodex is used to treat swelling, pain, and sprains.
The use of iodine containing salt has now become common. Deficiency of iodine causes many diseases related to thyroid gland.
Iodine is used to make many other important organic compounds in inorganic compounds.
It is also used as a reagent in the laboratory.
It is used by quantitative analysis to determine the concentrations of many substances in solutions.
It is also used in making colours, in color photography and in making dyes.