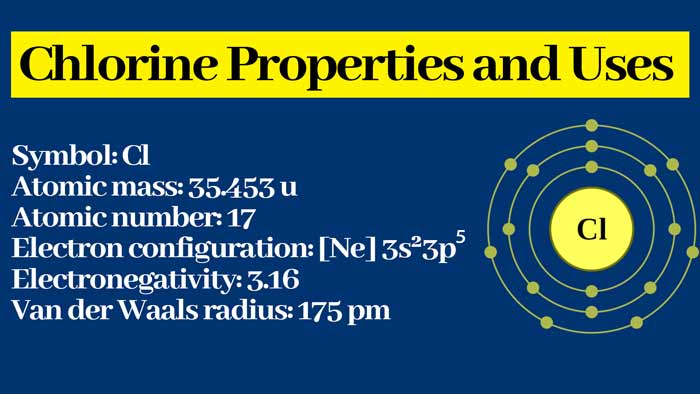
Chlorine Property : Physical and Chemical Properties | Uses
Chlorine Property : Physical
It is a greenish-yellow color with a pungent odor and a poisonous gas.
It is soluble in water. Its aqueous solution is called chlorine water.
It is heavier than air and oxygen.
It changes into liquid state at -34.5°C and into solid state at –101.5°C.
Chlorine Property : Chemical
Flammability
Chlorine gas does not burn on its own but helps in burning some substances.
Example: Putting a piece of burning sulfur in a jar filled with chlorine gas and closing the jar, it continues to burn.
2S + Cl2 → S2Cl2 (Sulfur Monochloride)
Reaction with non-metals
It reacts with most of the non-metals to form their chlorides.
Example: With sulfur it forms sulfur mono chloride.
2S + Cl2 → S2Cl2 (Sulfur Monochloride)
When white phosphorus is poured into a jar filled with chlorine gas, it rises and chlorides of phosphorus are formed.
2P + 3Cl2 → 2PCl3 (phosphorus tri-chloride)
2P + 5Cl2 → 2PCl5 (phosphorus penta-chloride)
- Chlorine Property : Physical and Chemical Properties | Uses
- Solar Energy : Light Waves, Reactions and Uses
- Chlorine Gas – Laboratory and Industrial Preparation
- Sulfuric Acid : Chemical Properties, Uses and Structure
- Rigid Body: Moment of Force or Torque formula
In the presence of sunlight with hydrogen it reacts with great intensity or explosion and forms hydrogen chloride gas.
H2 + Cl2 → 2HCl
Reaction with metals
Almost all metals react with chlorine to form their chloride salts. In these reactions chlorine gas is passed over metals in their hot states.
2Al + 3Cl2 → 2AlCl3
Cu + Cl2 → CuCl2
2Na + Cl2 → 2NaCl
Zn + Cl2 → ZnCl
Mg + Cl2 → MgCl2
Reaction with Water
Chlorine is soluble in water and its aqueous solution is called chlorine water. By keeping chlorine water in strong sunlight or in the presence of catabolists, the action of chlorine and water starts and oxygen gas is obtained.
Cl2 + H2O → HCl + HClO (Hypochlorous Acid)
HClO → HCl + O
O + O → O2
Reaction with sodium hydroxide
When passed in cold and dilute sodium hydroxide solution, it forms sodium hypochlorite(NaOCl).
Cl2 + 2NaOH → NaCl + NaOCl + H2O
On passing through hot and concentrated sodium hydroxide solution, it forms sodium chlorate(NaClO3).
3Cl2 + 6NaOH → NaClO3 + 5NaCl + 3H2O
Reaction with slaked lime
When flown over dry slaked lime, it forms bleaching powder.
Cl2 + Ca(OH)2 → CaOCl2 + H2O
Reaction with ammonia
It reacts with ammonia to form ammonium chloride and nitrogen.
2NH3 + 3Cl2 → N2 + 6HCl
6NH3 + 6HCl → 6NH4Cl
XXX ======= XXX ======== XXX
3Cl2 + 8NH3 → 6NH4Cl + N2
- Chlorine Property : Physical and Chemical Properties | Uses
- Solar Energy : Light Waves, Reactions and Uses
- Chlorine Gas – Laboratory and Industrial Preparation
- Sulfuric Acid : Chemical Properties, Uses and Structure
- Rigid Body: Moment of Force or Torque formula
- Preparation of Sulphuric Acid by Contact process with Reaction
- Preparation of Sulfuric Acid by Lead Chamber Process
If this excess amount of chlorine is processed with less amount of ammonia, then nitrogen trichloride(NCl3) is formed which is a very explosive substance.
3Cl2 + NH3 → NCl3 + 3HCl
Oxidizing Properties
Chlorine is a strong oxidising agent and it oxidizes various substances.
Example:
potessium bromide into bromine
2KBr + Cl2 → 2KCl + Br2
Potassium iodide is converted into iodine
2KI + Cl2 → 2KCl + I2
hydrogen sulfide to sulphur
H2S + Cl2 → 2HCl + S
The sodium sulfite solution is converted into sodium sulfate
Na2SO3 + Cl2 + H2O → Na2SO4 + 2HCl
ferrous sulfate to ferric sulfate
Cl2 + 2FeSO4 + H2SO4 → Fe2(SO4)3 + 2HCl
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
Aqueous solution of sulfur dioxide to sulfuric acid
SO2 + 2H2O + Cl2 → 2HCl + H2SO4
Chlorine Property : Addition Compounds
It forms addition compounds with carbon monoxide, sulfur dioxide and unsaturated carbolic compounds.
CO + Cl2 → COCl2 (Carbonyl chloride – Phosgene)
SO2 + Cl2 → SO2Cl2 (Sulfuryl Chloride)
C2H4 + Cl2 → C2H4Cl2 (Ethylene Dichloride)
Chlorine Property : Substitution Reactions
The reactions in which one or more hydrogen atoms of a compound are replaced by other atoms or groups are called substitution reactions. Chlorine exhibits substitution reactions with various carbonic compounds.

example :
CH4 + Cl2 → CH3Cl + HCl
Bleaching Properties
Being a strong oxidising agent, it is also a strong bleach. It spoils the color of wet flowers, leaves etc.
In this process, first this moisture reacts with H2O to produce nascent oxygen and then this nascent oxygen reacts with the colored components of flowers, leaves, etc. to oxidize them and make them colourless.
H2O + Cl2 → 2HCl + O
Coloured component + O → Colourless Component
Chlorine Uses
Chlorine gas is used in the manufacture of important compounds like bleaching powder, bromine, chloroform, carbon tetra chloride, phosjine, etc.
It is used for the purification of drinking water as a disinfectant.
Chlorine gas and chlorine water are used as a reagent in the laboratory.
It is also used in metal extraction and refining.
It is also used in making dyes and explosives.
It is used to make phosjin and musterd gas. Both these gases are very toxic. Musturd gas was used in the First World War. The composition formula of musturd gas is as follows –
Chlorine gas is used as a bleach for bleaching paper, clothing, etc.