Adsorption : Adsorption rate, Physisorption and Chemisorption
Adsorption Meaning
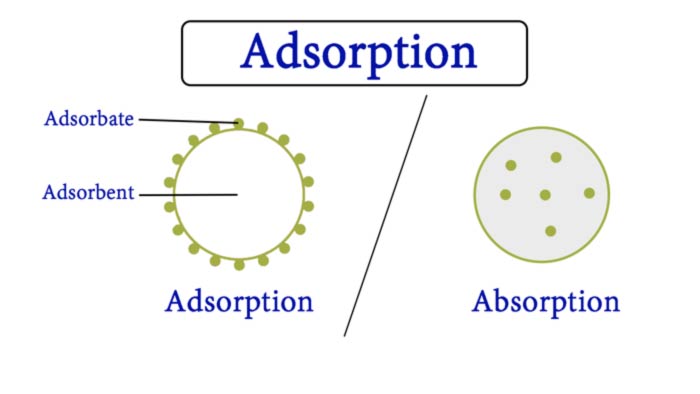
Adsorption (Latin: adsorbere = to suck in) means the accumulation of particles (atoms, molecules, ions, etc.) of one or more types of particles from a liquid or
gaseous phase on the surface of a solid or a liquid.
Since the liquid or gaseous substance accumulates on the surface, there is always a change in concentration at the phase interface during this process. Adsorption is usually spoken of when there is a gas/solid interface.
Adsorbent
A substance that accumulates (adsorbs) particles from a neighboring gaseous or liquid phase at its interface is called an adsorbent or substrate.
- Adsorption : Adsorption rate, Physisorption and Chemisorption
- Electric Cell : E.M.F., Terminal Potential, Internal Resistance
- How Transistor Works : PNP and NPN Transistors
- How p-n Junction Diode works : Forward and Reverse Biasing
- Semiconductors : How Semiconductor works and Types
- X-Rays – Production, Properties, Wavelength and Uses
Adsorptive
The particles that are in the adjacent gaseous or liquid phase and accumulate on the adsorbent are referred to as adsorptive.
Adsorbate
A particle attached (adsorbed) to the surface of an adsorbent is called an adsorbate. The combination of the adsorbent with the substance adsorbed on it (adsorptive) is often referred to as an adsorbate.
Degree of coverage
The surface of an adsorbent contains a certain number of adsorption sites. The quotient of the number of occupied adsorption sites divided by the number of available adsorption sites is described by the degree of coverage Θ.
Θ = Number of occupied adsorption sites / Number of existing adsorption sites
The degree of coverage Θ is therefore a measure of the adsorption on a surface.
Θ=0: all places are free
Θ=1: all places are occupied
The degree of coverage can also be specified as a percentage.
Adsorption Rate
The rate at which a surface is covered by the adsorbent depends on how fast the adsorbent can absorb the energy of the impacting particle. If this happens too slowly, the particle will be pushed back into the gas phase. So not all the particles hitting the surface of the adsorbent are adsorbed.
The proportion of collisions that result in adsorption is called the adsorption probability.
adsorption probability = frequency of adsorption of particles /frequency of impact of particles
The probability of adsorption decreases with increasing degree of surface coverage.
Physisorption
Physisorption is based on the effect of van der Waals forces between adsorbate and adsorbent. Although these interactions are weak, they extend over large distances, since adsorption on the existing adsorbate layer is also possible due to van der Waals forces, and several adsorption layers can form in this way.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
When a particle approaches a surface, two opposing forces act on this particle: a repulsive force and an attractive force. The magnitude of these forces depends on the distance r between the particle and the surface.
The repulsive force is proportional to r−12 while the attractive force is proportional to r−6. There is a superimposition of the forces of attraction and repulsion acting on the particle, the sum of which leads to the following expression for the potential energy of the particle:
E(r) = −B⋅r−6 + A⋅r−12
The function obtained is called the Lennard-Jones (6,12) potential.
The adsorption enthalpy of physisorption is of the order of the condensation enthalpy, typical values are around 20 kJ/mol. This energy is not enough to break bonds, so physisorption keeps a molecule as such.
Chemisorption
Chemisorption is based on the effect of much stronger binding forces, the strength of which is similar to that of chemical bonds. These interactions are significantly stronger than in the case of physisorption, but the surface can only be covered with a monolayer.
In contrast to physisorption, a chemisorbed molecule can decompose by binding with the surface atoms. For example, hydrogen is adsorbed on the surface of transition metals not in molecular but in atomic form.
The adsorption enthalpy of chemisorption is in the range of about 200 kJ/mol.
Desorption
Desorption is the inverse of adsorption. Energy must be supplied for desorption, since the adsorbed particles have to be raised from the potential minimum that they have reached through adsorption.
In the case of physisorbed particles, only a small addition of energy is required for desorption because of the low strength of the bond between adsorbent and adsorbate.
Therefore, small increases in temperature are usually sufficient to remove the particles from the surface. In the case of chemisorbed particles, on the other hand, bonds have to be broken, so that activation energy has to be applied here.