Bleaching Powder : Preparation, uses of Bleaching Powder
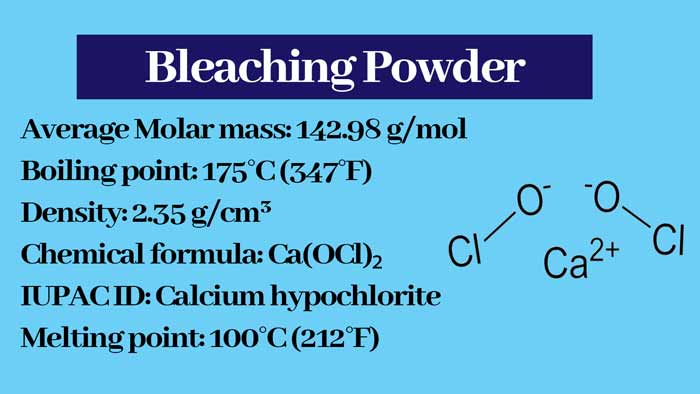
Bleaching Powder : Calcium hypochlorite
One molecule of bleaching powder (CaOCl2) contains one calcium ion (Ca2+), one chloride ion (Cl–) and one hypochlorite ion (OCl–). Hence it is also called Calcium Chloro Hypochlorite. It is a mixed salt.
Preparation of Bleaching Powder
Bleaching powder is made by the reaction of chlorine gas on dry slaked lime.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
There are two main methods of making it in commercial quantity –
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
Hasenclever Method
The plant used in this method is shown below. This plant has many hollow stems. In which many stirrers are fitted in the rods in the middle. These cylinders are related to each other in such a way that the substances filled in them can come into each other.

When slaked lime is poured from the upper cylinder, chlorine gas is passed through the lower cylinder and on running the stirrers, the reaction of chlorine and slaked lime takes place and becomes bleaching powder.
Bachmann’s method
The plant used in this method is shown below. This plant is in the shape of a tower, in which there are many rakes attached to a rod in the middle.

In this, slaked lime is poured from above with the help of compressed air and chlorine gas and hot air is blown from below.
The bleaching powder is produced by the action of chlorine and slaked lime in the plant which keeps coming out from the underside of the plant.
Bleaching Powder Properties
It is a light yellow powder. It is soluble in water in small quantities and it smells like chlorine.
Bleaching powder slowly decomposes on its own and forms Calcium Chloride and Calcium Chlorate.
6CaOCl2 → 5CaCl2 + Ca(ClO3)2
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
It reacts with carbon dioxide to form chlorine gas. Because of this, it smells like chlorine.
CaOCl2 + CO2 → CaCO3 + Cl2
It also releases chlorine gas by reacting with dilute acids.
CaOCl2 + H2SO4 → CaSO4 + H2O + Cl2
CaOCl2 + 2CH3COOH → Ca(CH3COO)2 + H2O + Cl2
It reacts with water to form nascent oxygen which acts as a bleaching agent.
2CaOCl2 + 2H2O ⇌ CaCl2 + Ca(OH) + 2HClO
HClO ⇌ HCl + O
colored substance + O → colorless substance
It decomposes to form oxygen gas even in the presence of cobalt chloride (catalyst).
2CaOCl2 → 2CaCl2 + O2
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
- Iodine : Properties, Preparation and uses
- Bromine – Preparation, Physical and Chemical Properties a Uses
- Chlorine Property : Physical and Chemical Properties | Uses
Uses of Bleaching Powder
Bleaching powder reacts with certain compounds present in various substances to form chlorine or nascent oxygen which, being a strong oxidising agent, removes the color of those substances.
Therefore, bleaching powder is used in the bleaching of colors of various materials (clothing, furniture, paper, etc.). That is why it is called Bleaching Powder.
It is also used as a bactericide and in the preparation of some compounds.
Purity of Bleaching Powder
When bleaching powder is reacted with more amount of dilute acids, chlorine gas is obtained, which is available chlorine gas.
To determine the purity of a sample of bleaching powder, the amount of chlorine available is often estimated. The free chlorine in ordinary bleaching powder is 35% (by weight).
If the available chlorine content is less than 35%, then the bleaching powder will be impure or have been kept open for a long time.
- Preparation of Aldehydes and Ketones : Chemistry Page
- Arsenious Oxide : Preparation, Properties and Uses
- How to find Equivalent Weight in Chemistry ? Chemical Formula
- Oxidation Number : How to find Oxidation State
- Bleaching Powder : Preparation, uses of Bleaching Powder
- Iodine : Properties, Preparation and uses
- Bromine – Preparation, Physical and Chemical Properties a Uses
- Chlorine Property : Physical and Chemical Properties | Uses
To estimate the amount of chlorine available in bleaching powder, it is treated with dilute hydrochloric acid or high amounts of glaciual acetic acid.
CaOCl2 + 2HCl → CaCl2 + H2O + Cl2
CaOCl2 + 2CH3COOH → (CH3COO)2Ca + H2O + Cl2
The above reaction is carried out in the presence of KI. Therefore, chlorine obtained from the above reaction reacts with KI to liberate iodine.
Cl2 + 2KI → 2KCl + I2
I2 + KI ⇌ KI3(dark Yellow )
Titration with hypo solution is done by adding few drops of starch to this solution. The color of the solution is dark blue before the titration due to formation of starch iodide, but the solution becomes colorless due to the use of iodine in the reaction at the end point.
I2 + 2Na2S2O3 → 2NaI + Na2S4O6
By using the data obtained from the above experiment and by calculation, we find the amount of chlorine available in bleaching powder.
- Importance of Biomolecules in Life || What are the 4 main biomolecules?
- Valency of Elements || How to Find Valency || What is the Valency of the atom?
- Resonance effect or mesomeric effect || What is resonance effect with example?
- Glucose Structure: Physical and chemical properties, Glucose Chemical Reaction
- Introduction of Inductive-Effect || How does Inductive Effect Work?
- IUPAC Name : How to find the IUPAC name of compounds.
- What is Urea || How to make Urea Fertilizer, || Urea uses
- Sodium Chloride Properties || Why Sodium Chloride is Soluble in Water
Let’s assume that:
Weight of bleaching powder = W gram
Used volume of hypo solution = V ml
Normality of hypo solution = N
The number of milli equivalents in SO-chlorine = the number of milli equivalents in iodine
= number of milli equivalents in Hypo
= N x V
Amount of chlorine = Milli equivalents x (1/1000) x Equivalent weight
= N x V x (1/1000) x 35.5
= 0.0355 x N x V
Percentage amount of chlorine available (by weight) = (0.0355 x N x V/W ) x 100